The treatment of cancer of the supraglottic larynx is in a continual state of evolution. Clinicians are anxious to provide pa-tients with the best possible functional outcome while controlling tumour. Therapeutic options for patients with cancer limited to the supraglottic region of the larynx include transoral microsurgical resection, chemoradiation therapy, open horizontal supraglotic laryngectomy and supracricoid larygectomy.
Supraglottic laryngectomy was first introduced to the United States of America over 50 years ago. It effectively controls limited cancer involving the supraglottic portion of the larynx. The overwhelming majority of patients undergoing supraglottic laryngectomy can swallow by mouth 10-14 days after surgery. However, the risk of aspiration pneumonia is significant in patients with inadequate cough and poor pulmonary reserve. It is therefore not appropriate for patients with cardio-pulmonary compromise which is so significant that they cannot temporarily tolerate moderate amounts of aspiration. This group of patients should not be offered this procedure.
All patients with cancer of the supragloticlarynx require careful pre-treatment evaluation which should include laryngoscopy as well as imaging. The ideal candidates are those patients with tumour confined to the supraglottic larynx without involvement of either arytenoid. Cervical metastatic disease is highly prevalent for cancers of the supraglottic cancers. This risk is to both sides of the neck inasmuch as the larynx should be considered a midline structure. Routine bilateral functional neck dissection of Levels 2, 3, and 4 is therefore advocated for all patients treated with surgery. Patients will bulky or bilateral metastases at presentation are considered a relative contraindication to supraglottic laryngectomy because of the predictable need for postoperative adjuvant radiation. Most patients can tolerate radiation therapy after supraglottic laryngectomy, however, it almost invariably compromises function with increased risk of aspiration.
Resection of tumour extension from the aryepiglottic fold to the medial wall of the pyriform sinus can be accomplished with modifications to become a partial laryngopharyngectomy. Tumour extension to the vallecula and tongue base can be accommodated, but this kind of tumour involvement will require partial tongue base resection, which in turn, will compromise functional outcome. Additionally, tongue base involvement almost always will call for postoperative irradiation, which, once again, is poorly tolerated after supraglottic laryngectomy.
The only absolute contraindication to supraglottic laryngectomy is spread through the paraglottic space inferiorly, such that the plane of resection through the ventricles would result in residual tumour or spread to involve both arytenoids. Under these circumstances, therapeutic alternatives include chemoradiation therapy or total laryngectomy.
The patient’s cardiopulmonary function is an essential criterion which requires evaluation when considering supraglottic laryngectomy. All patients require temporary tracheotomy which allows the treatment team to provide suctioning and to help treat aspiration. However, patients with poor pulmonary reserve should not undergo supraglottic laryngectomy. There are no concrete, objective guidelines for eligibility such as arterial blood gases or spirometry. In general, patients with good functional capacity such that they can walk up a flight of stairs comfortably, can tolerate a supraglottic laryngectomy. Patients who cannot ambulate will, in general, not tolerate supraglottic laryngectomy.
The procedure is done under general anaesthesia. A prophylactic antibiotic is administered.
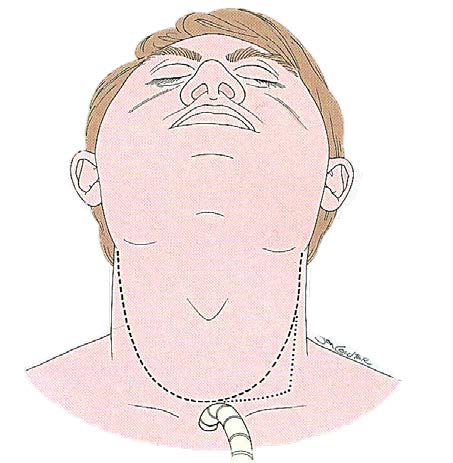
The patient is positioned in the supine position with the neck slightly extended on a shoulder roll. A superiorly based apron flap is planned such that the horizontal portion of the apron overlies the second or third tracheal ring (Figure 1). The apron flap is elevated just underneath the platysma muscle.

Tracheotomy is then accomplished. Under most circumstances this is facilitated by division and ligation of the thyroid isthmus.
Bilateral neck dissection is done in all patients. In the absence of identifiable pathologic adenopathy, elective neck dissection should remove the fatty lymphatic aponeurosis in Levels 2, 3 and 4. Level 2b superior to the spinal accessory nerve need not be dissected. The spinal accessory nerve, jugular veins, and sternocleidomastoid muscles are preserved.
The suprahyoid strap muscles are released from the superior surface of the hyoid bone with diathermy. The infrahyoid strap muscles are subsequently released approximately 1 cm below the inferior insertion of the muscles to the hyoid bone and reflected inferiorly to expose the thyroid cartilage.
The external perichrondrium of the thyroid cartilage is then released along the superior and lateral aspects of the thyroid laminae such that it can be reflected inferiorly (Figure 2).
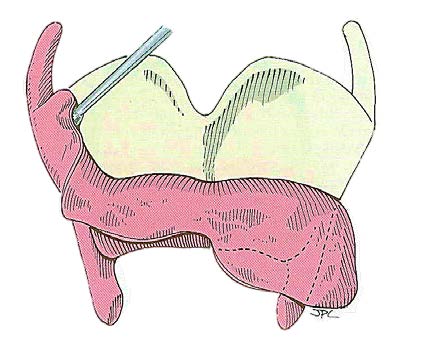
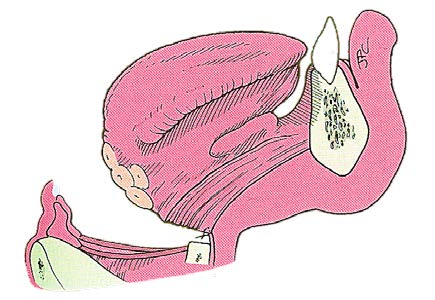
The cartilage is then cut horizontally with an oscillating saw (Figure 3), recalling that the intention is to resect the larynx through the ventricle (Figure 4). This requires that the cut be made approximately midway between the thyroid notch and the lower border of the thyroid cartilage. The cartilage cut may be slanted laterally toward the superior cornu on the less involved side which allows for preservation of the constrictor insertion
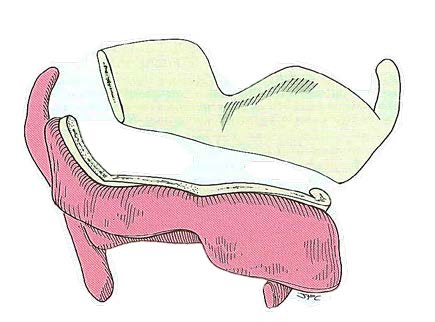
Pharyngotomy is done through the vallecula when tumour is confined to the supraglottic larynx. This allows subsequent mucosal cuts to be made under direct vision.
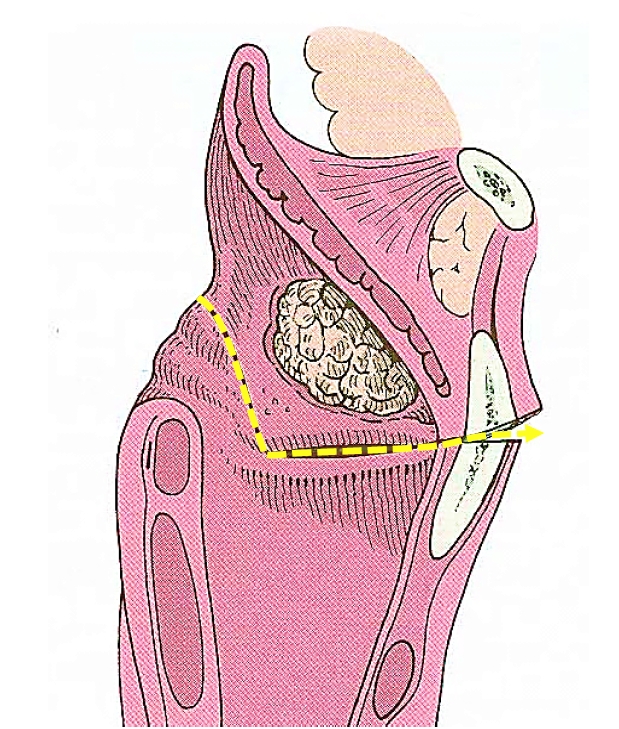
Mucosal incisions are made laterally along the aryepiglottic folds and then across the face of the arytenoids to resect the entire epiglottis and false vocal folds. The inferior cuts are made horizontally through the laryngeal ventricles to connect the mucosal cuts with a cartilage cut (Figure 4).
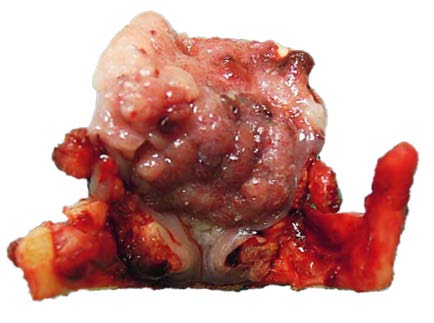
The resected specimen is carefully evaluated and frozen sections done to assure complete tumour removal (Figure 5).
Haemostasis must be complete. This may be especially difficult along the cut edge of the tongue base where intermittent suture ligatures are recommended.
A nasogastric tube is inserted before the closure is accomplished.
Reconstruction starts with approximation of the tongue base to the glottic larynx in such a way that the tongue base is set back over the glottis to reduce the potential for aspiration (Figure 6). This requires that the yellow fibrofatty aponeurosis of the tongue base be directly approximated to the external perichrondrium of the thyroid cartilage. If difficulty is observed with tearing of the external perichrondrium, the sutures may be directed through residual thyroid cartilage. This initial layer of closure is then reinforced by approximating the suprahyoid musculature to the infrahyoid muscles. The closure is carried laterally to assure there is no evidence of salivary leak in the area of the pyriform sinus.
After closure, suction drains are placed in either side of the neck and over the mucosal suture line and brought out through separate stab wounds.
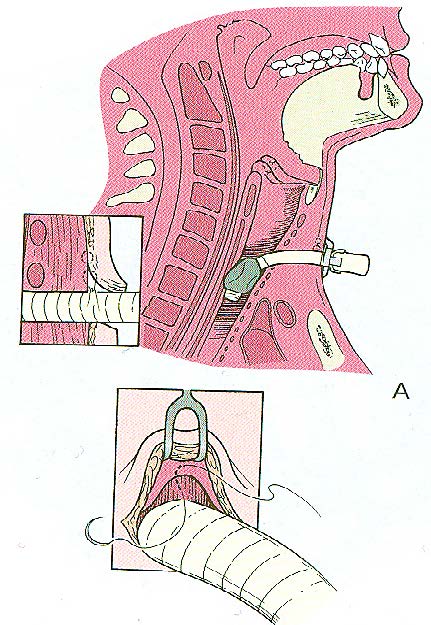
The apron flap is then redraped. Great care is taken to assure that the tracheotomy site is isolated from the potential spaces in both sides following neck dissection by suturing (Figures 7A & B). Failure to isolate the tracheotomy from the potential space created by the neck dissection may result in transmission of contaminated mucus from the trachea underneath the cervical flap and subsequent wound infection.
The anaesthesia tube is removed and replaced with a cuffed tracheotomy tube.
Care is taken to assure the integrity of the suction drains. Routine tracheotomy care is instituted. All patients are ambulated as soon as possible to assure restoration of tidal volume and prevention of atelectasis.
The tracheotomy tube can be removed when the patient demonstrates that he/she can protect the airway and is no longer aspirating. This usually takes 7-14 days. One practical method is to deflate the cuff of the tracheotomy tube when the patient is seated. Most patients will have some temporary aspiration which can be suctioned. Thereafter it should be determined if the patient can tolerate an uninflated tracheatomy tube without excessive coughing and aspiration for 24hrs.
Having achieved this milestone, the cuffed tracheotomy tube should be removed and replaced with a smaller tracheotomy tube without a cuff, which can be plugged so that the patient breathes past the tube and through the larynx, to ascertain if the patient’s airway is satisfactory to allow decannulation.
The overwhelming majority of patients will tolerate an oral diet best when the tracheotomy site has healed. Introduction of an oral diet before the tracheotomy site is closed is difficult because the tracheatomy is associated with a less effective cough, tethering of the larynx, and diminished proprioception such that aspiration is more likely.
Following recovery from the acute phases of healing, supraglottic laryngectomy patients have remarkably good voice with few limitations to diet.
Recovery is significantly, and sometimes severely, challenged when postoperative irradiation therapy is required. Radiation causes lymphoedema and stasis, which compromises swallowing and may cause airway obstruction. Therapeutically induced obstructive sleep apnoea has been reported under these circumstances.
Patients who have had prior irradiation therapy can tolerate supraglottic laryngectomy. However, the healing is slower, recovery of deglutition is prolonged and some element of chondroradionecrosis of the residual thyroid cartilage may be observed. All of these factors can be treated and a successful functional outcome will be achieved in properly selected patients.
Jonas T. Johnson, M.D.
The Dr Eugene N. Myers Professor and Chairman
Department of Otolaryngology
Eye and Ear Institute
200 Lothrop Street
Suite 500
Pittsburgh, PA 15213
USA
johnsonjt@upmc.edu
Johan Fagan MBChB, FCORL, MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town
South Africa
johannes.fagan@uct.ac.za