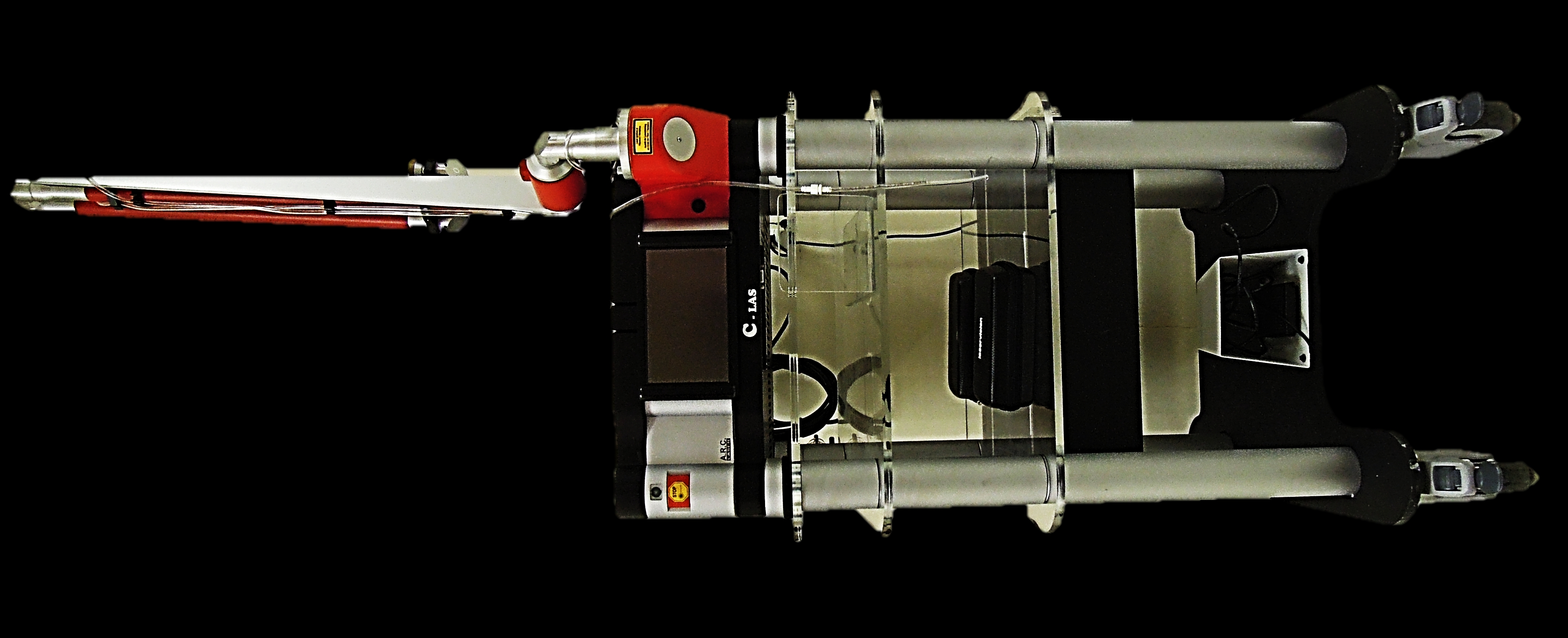
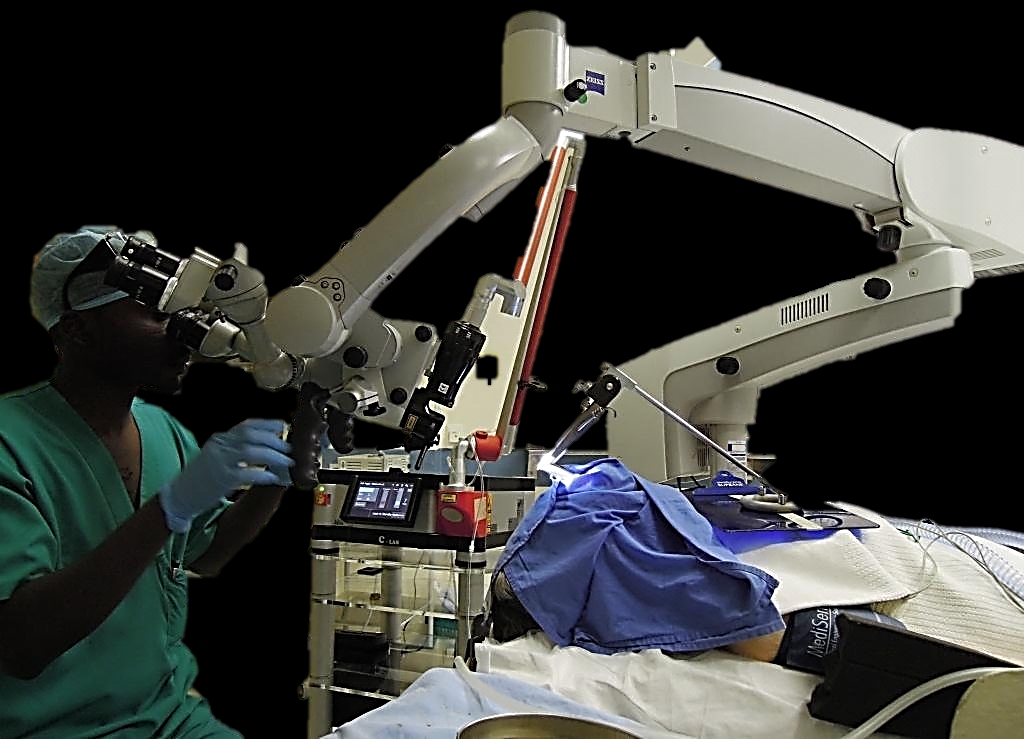
CO2 laser is used to resect or vaporise benign and malignant lesions of the upper aerodigestive tract. This chapter focuses on transoral CO2 laser technique using a micromanipulator attached to an operating microscope. The advantages of using CO2 laser with an operating microscope are microsurgical precision, excellent intraoperative detail, and a dry surgical field; the functional outcomes in terms of swallowing and speech exceed that of traditional external surgical approaches.
Surgeons must familiarise themselves with the laser machine, its settings and delivery systems, and its tissue effects prior to attempting to use it clinically.
Surgical lasers convert radiation energy into heat at the point of contact of the focused radiation with the tissue. The laser beam is generated in a gas-containing discharge tube. The light beam is collimated (coherent), is of a single wave-length (monochromatic), and can be reflected and hence manipulated by mirrors, and focused by passing it through lenses. Because CO2 laser falls outside the visible light spectrum and is therefore invisible, the laser machine generates a red diode laser aiming beam that is focused at the same working distance as the microscope to indicate to the surgeon where the beam is directed. The laser beam is directed along a spring-loaded
articulated arm which connects to a beam applicator. The articulated arm has mirrors at each articulation; therefore one has to handle the articulated arm with great care so as not to disturb the alignment of the mirrors. The laser is fired when the foot pedal is depressed.


The laser beam exits the end of the articulated arm and is delivered to the tissue via a handpiece, laser bronchoscope or a micromanipulator attached to an operating microscope (Figures 2, 3, 4).
Handpiece (Figure 2): This is generally used for excising or vaporising skin lesions or lesions in the oral cavity and oropharynx. Suction tubing is attached to a suction port located on the side of the instrument to extract smoke and laser plume. The tip extension maintains the correct distance from the tissue to keep the laser beam correctly focused.
/Fig%202.png)
/Fig%203.png)
/Fig%204.png)
Micromanipulator (Figures 4, 5): The micromanipulator has a mirror located in the view path of the surgeon. The surgeon directs the laser beam precisely onto the desired tissue target. The coarse and fine focus settings are used to focus or defocus the laser beam and to set the spot size.

Laser settings are selected according to the type of tissue to be lasered (cartilage / muscle / mucosa), the desired depth of the laser cut, the need for haemostasis, and the need to avoid excessive heating of tissue. The surgeon can optimise the benefits of CO2 laser by adjusting:
Figure 6 illustrates a typical control panel of a laser machine. Settings available to the surgeon (left-to-right) include continuous vs. pulsed beam, lengths of pulses (milli-seconds), time intervals between pulses, brightness of the aiming beam; power (Watts); and “Laser standby” or “Laser ready” settings. Spot size is set with the focus setting of the micromanipulator.

Laser Power Density (PD) is a key surgical parameter. It is affected by the distance from the tissue, the diameter of the beam (laser spot size), and the number of Watts (Joules/sec); all these parameters can be controlled by the surgeon.
Key formulae to remember are:
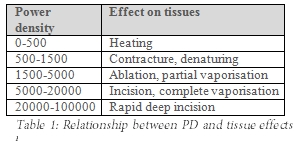
By controlling PD the surgeon can maximise the benefits of CO2 laser surgery (Table 1).
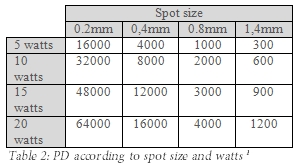
To achieve complete vaporisation, the surgeon should aim for 4500 PD at the tissue interface. Table 2 illustrates how critical the number of Watts and laser spot size are to PD
This is selected by the surgeon every time the laser machine is used, and can be pre-programmed for different types of surgery. A laser machine with power settings of up to 40W is recommended for head and neck surgery.
The smaller the spot size, the less the range of depth of focus at which the laser cuts effectively and the more accurately the laser has to be focussed. Spot sizes of 0.5-0.8mm provide a comfortable compromise between depth of focus and cutting ability.
To coagulate bleeding vessels, PD has to be adjusted to a level at which tissue is no longer vaporised, but is only heated and blood vessels are coagulated (Table 1); Tables 2 illustrates how increasing spot size by defocusing the laser beam reduces the PD.
The smaller the spot size, the less the range of depth of focus at which the laser cuts effectively and the more accurately the laser has to be focussed. Spot sizes of 0.5-0.8mm provide a comfortable compromise between depth of focus and cutting ability.
To coagulate bleeding vessels, PD has to be adjusted to a level at which tissue is no longer vaporised, but is only heated and blood vessels are coagulated (Table 1); Tables 2 illustrates how increasing spot size by defocusing the laser beam reduces the PD.
The total duty cycle is the total time that laser energy interacts with target tissues.
This can be regulated in a number of ways (Figure 6):
CO2 laser is absorbed almost completely by intracellular water and causes vaporisation of water and cells. Because 99% of heat that is generated is lost in the vapour that is released, peripheral tissue injury and necrosis are limited to <0.01mm; this allows one to preserve laryngeal function, to limit postoperative swelling and pain, and for pathologists to be able to interpret resected tissues for margins
Laser fire is a very rare but potentially fatal complication of transoral laser microsurgery. Special care has to be taken to avoid this catastrophe (Table 3). CO2 laser is invisible, reflects off smooth surfaces, can cause photothermal injury to patients and staff; and can cause fires by igniting flammable materials such as drapes, plastics, oxygen, and flammable cleaning solutions. Fire may also occur if the beam ignites the endotracheal tube (only if FiO2 too high), or when “flaming” occurs when lasering carbonised tissue as when cutting through cartilage at high watts.

Because CO2 laser is absorbed and “neutralised” by water, wet swabs and patties are used and the cuff of the endotracheal tube may be filled with saline. To reduce the risk of airway fire, it is critical that the anaesthetist keeps the FiO2 at a minimum (+/- 30%) to avoid a blow-torch effect if the oxygen in the airways is ignited. It is advisable that an open bowl of water/ saline be kept at hand to douse a laser fire.
The principal anaesthetic challenges are to use endotracheal tubes that permit the surgeon to work in the confined space of the larynx and pharynx, and to eliminate the risk of laser fire (Table 3).
Gases: Avoid nitrous oxide if possible and maintain inspired oxygen FiO2 as low as is clinically feasible; this requires continuous monitoring of the patient’s oxygenation by pulse oximetry. The surgeon must notify the anaesthetist before activating the laser and give the anaesthetist enough time to reduce the delivered oxygen concentration to a minimum, to stop using nitrous oxide, and then to wait a few minutes for the oxygen concentration in the airways to drop to a safe concentration.
Airway: The airway may be maintained in a number of ways:
anaesthetic gases administered through the suction port of the laryngoscope
It is important that the surgeon discusses with the anaesthetist the optimal method of maintaining an airway for the specific patient e.g. nasotracheal intubation may be best for base of tongue cancer, but is problematic for an endolaryngeal tumour as it fixes the position of the endotracheal tube.
All tubes are flammable; no tube is therefore “safe”. The problem is not the type of tube, but perforating the tube when the O2 concentration in the tube is too high. The authors use regular PVC tubes, but take special care to protect them with a strip of wet cloth cut from e.g. surgical drapes (Figure 7), or with neuro patties.
/Fig%207.jpg)
The drawbacks of “laser tubes” are that they have thicker walls and are expensive. PVC tubes with 5mm inner diameter are more flexible, less expensive and pose no increased risk for fire as long as the FiO2 is <30%. The cuff of the tube may be filled with saline tinted with methylene blue to act as a marker for cuff puncture, and to flood the airway with saline to prevent a fire when the cuff is punctured by the laser. The 2nd author (W.S.) however prefers to fill the cuff with air because it is easier to rapidly extubate a patient in case of emergency, and to avoid fluid running into the bronchi when a fluid-filled cuff is perforated.
To prevent reflection of the laser beam, instruments with beaded or mat surfaces are used (Figure 8). Adequate tumour exposure is critical to TLM; an integrated suction channel to remove smoke is essential. It is therefore crucial that a variety of laryngoscopes and pharyngoscopes is available (Figure 8).

/Fig%209.jpg)
/Fig%2010.png)
/Fig%2011.jpg)
/Fig%2012.png)
/Fig%2013.png)
/Fig%2014.png)
A typical setup has the anaesthetic machine placed at the patient’s feet; this requires extensions for anaesthetic tubing and intravenous lines. A camera is attached to the microscope so that the assistant and nurses can follow the procedure on a monitor. Two suction systems are required, one attached to the laryngoscope to extract smoke from the surgical field and the other attached to the hand-held suction tube. Monopolar diathermy should be available for laser procedures other than mid-cord T1 glottic carcinomas.
Surgical exposure is a critical element of TLM. It may prove sometimes impossible to proceed with TLM due to inadequate access
/Fig%2015.png)
/Fig%2016.png)
CO2 laser may be used to incise, excise or vaporise (ablate) tissue. Novices should start by operating on simpler cases e.g. smaller tumours of the aryepiglottic fold, supraglottis, or medial wall of piriform fossa. Important principles of CO2 laser surgery include:

/Fig%2018.jpg)
14. Bread slicing: (Figure 19) In order to ensure an adequate oncological deep margin, but to minimise impairment of voice quality by not resecting too much tissue, the surgeon needs to precisely determine the tumour depth and to resect along the deep aspect of the tumour under high magnification. This is achieved by serially sectioning (bread slicing) the tumour. This is applicable to all tumours other than very superficial (Tis or T1) tumours which are simply peeled off the vocal ligament staying in Reinke’s space
15. Orientating specimens: Take special care not to lose the orientation of specimens when removing them from the patient. Specimens are pinned to small cut-outs of cork floor tiles that are placed in formaldehyde so that the pathologist can orientate the specimen (Figure 20).
/Fig%2019.png)
/Fig%2020.png)
16. Tumour topography: Include a detailed drawing on the pathology form and in the patient’s notes of the precise location of all the resected specimens
This is a controversial topic, and one that may cause surgeons a great deal of anxiety as to whether the patient should be returned to the operating room for an additional resection, whether simply to advise close surveillance, or whether to recommend adjuvant irradiation. Factors that play a role in reaching such a decision include tumour location, size, function (voice and swallowing), patient fitness, extent of the initial resection e.g. onto cartilage or carotid, not knowing precisely where the positive or close margin is located, and the reliability of regular follow-up. Frozen section may be useful with larger resections e.g. base of tongue and hypopharynx; yet pathologists are reticent to do frozen sections on small resections e.g. T1 glottic cancers, as it makes it difficult to comment definitively on adequacy of margins. The surgeon’s impression of the adequacy of a resection as seen through the microscope is also important; he/she may elect to adopt a watchful waiting approach even when tumour is reported to be “present at the margin”, with the knowledge that cells are denatured and killed adjacent to the incision during laser excision; such a watchful waiting approach applies especially to small mid-vocal fold carcinomas that can be carefully monitored and reoperated on timeously without adversely affecting prognosis.
The challenge for the laser surgeon is to strike the correct compromise between an oncologically adequate resection and preservation of voice quality. The new laser surgeon should initially select smaller tumours and progress to larger tumours only once he/she has gained a good understanding of the endoscopic “inside-out” surgical anatomy of the larynx, and has become proficient with laser surgery technique. We do not advise voice rest after surgery except for superficial defects of the membranous cord.
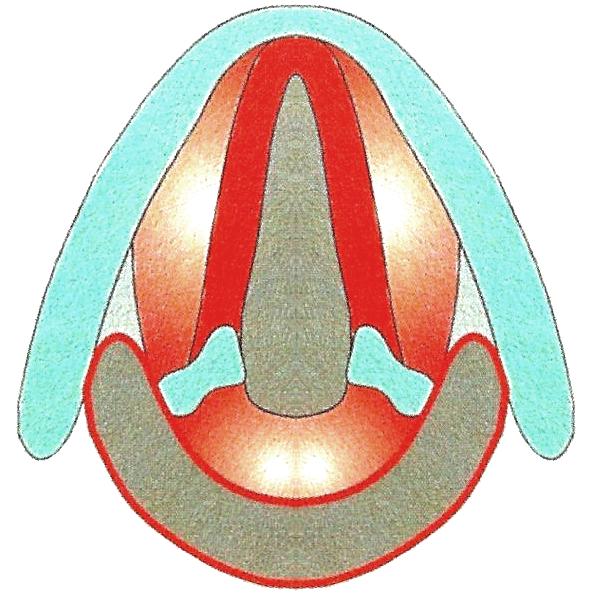
The European Laryngological Society proposed a useful classification of endoscopic cordectomy in 2000 (Table 3, Figure 21) 2.

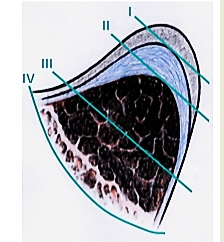
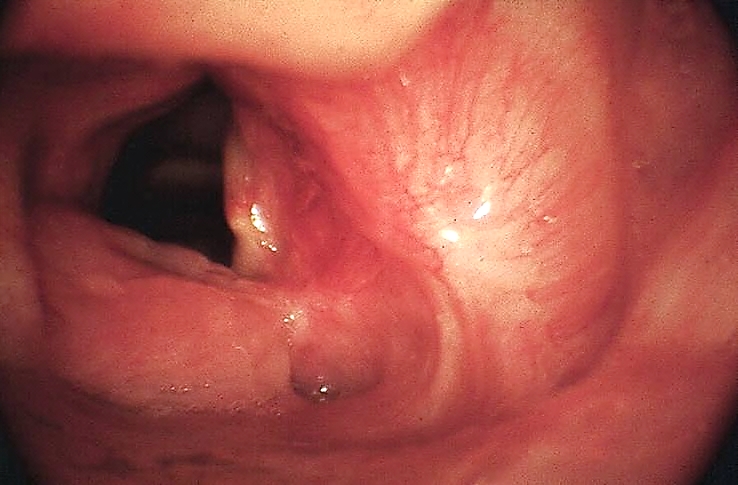
One can anticipate an excellent speaking voice following Type I or subepithelial resection of T1s and T1 cancers of the membranous vocal fold (Figures 22, 23).
/Fig%2022.png)
/Fig%2023.png)
Figures 24 & 25 illustrate T1 glottic cancers that required Type II cordectomy and subsequent “new cord” formation due to a scar band; such patients can be expected to have a good, though not quite normal quality voice.
/Fig%2024.png)
/Fig%2025.png)
Figure 26 illustrates transmuscular or Type III cordectomy for T2 glottic carcinoma. This defect is subsequently filled by a scar band leading to a very adequate speaking voice.
/Fig%2026.png)
With Type IV cordectomy the resection extends onto thyroid cartilage with loss of volume of the hemilarynx and less predict-table voice outcome (Figure 27).
/Fig%2027.png)
This applies to patients who undergo com-plete resection of the paraglottic space for T3 glottic carcinoma. Such patients may have to phonate using the false vocal cords and may produce a good voice; hence the value of preserving the false vocal cords if possible.
The objective is to do a Type 1 resection, and to restore a normal speaking voice. Resection margins of <1mm are acceptable, as with close follow-up, recurrences can be resected without affecting oncologic outcome. Set the laser at its smallest spot size, low power (1.5-3W) and CW superpulse mode; this allows a very precise dissection with minimum lateral thermal injury to the tissues. Surgery is done under high magnification. The initial incision is made only through epithelium (Figure 28).
/Fig%2028.jpg)
The cut edge of the epithelium is picked up with microforceps and the tumour is dissected off the vocal ligament, taking care to remove and pin the tumour to the cork without losing spatial orientation (Figure 20).
With deeper tumours requiring Types II-IV resections the objective is to do an adequate resection (>1mm margin), and to maintain an adequate speaking voice (Figure 29).
/Fig%2029.png)
Set the laser at its smallest spot size, power of 3-5 W and superpulse mode; this allows a very precise dissection with minor lateral thermal injury. Surgery is done under high magnification. It is imperative to bread-slice the tumour in order to determine the depth of the tumour and the deep resection plane, and to remove it in sections (Figures 18, 19, 30, 31).
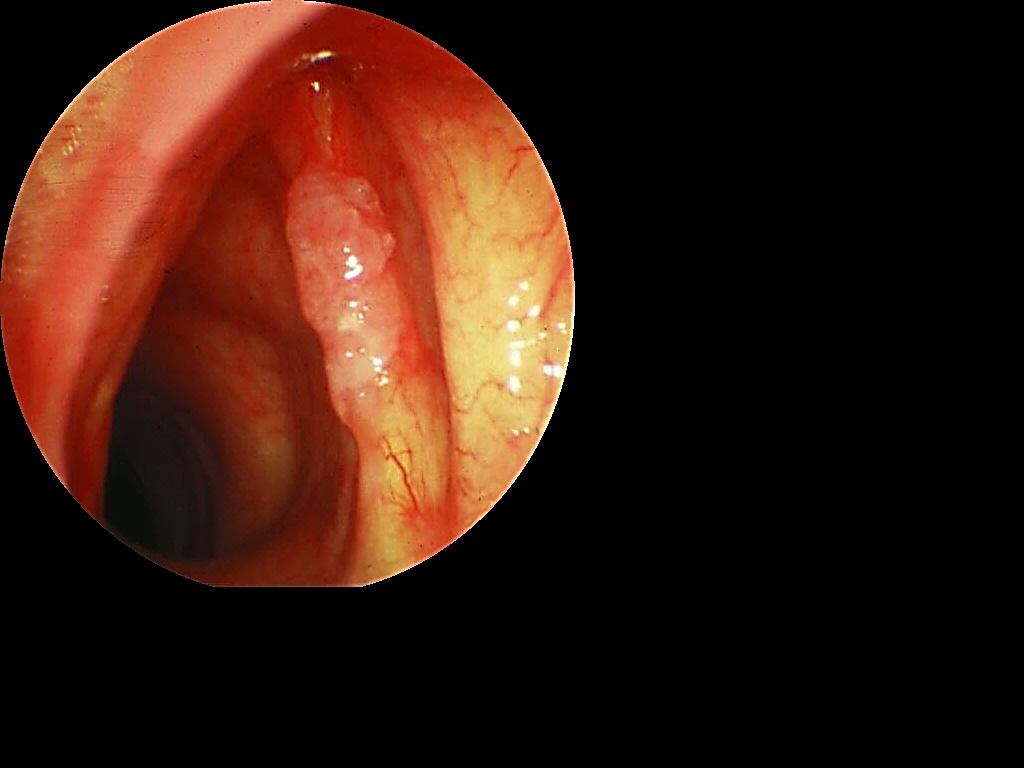
It is generally easiest to 1st remove the posterior segment, especially if access to the anterior commissure if poor (Figure 32).
/Fig%2031.png)
/Fig%2032.jpg)
Bleeding may be encountered especially when dissecting adjacent to, or below, the anterior commissure and lateral to the vocal process of the arytenoid. Small vessels may be coagulated by defocusing the laser beam. More brisk bleeding is controlled using monopolar or bipolar coagulation forceps. Avoid using suction diathermy on the vocal fold. Take care to remove and pin the tumour segments to the cork without losing spatial orientation, and to make a detailed drawing of the orientation of the segments in the patient records and on the pathology request form. It is also useful to paint the basal area (deep margin) blue, and to ask of the pathologist to determine whether the painted area is free of tumour.
The authors do not consider anterior commissure cancer to be a contraindication to laser resection. However some surgeons consider involvement of the anterior commissure to be a contraindication for laser resection and advocate vertical, supracricoid or even total laryngectomy.
The following needs to be considered when managing cancers of the anterior commissure with laser resection:
/Fig%2033.jpg)
/Fig%2034.png)
/Fig%2035.jpg)
The surgeon follows the tumour endoscopically. Thyroid cartilage may have to be resected with laser to improve exposure and for resection of extra-laryngeal tumour; resection may extend to just deep to the skin of the neck if needed (Figure 36)
/Fig%2036.jpg)
Webbing of anterior commissure causing poor voice (Figure 37): Webbing occurs when the anterior ends of both vocal cords are denuded or resected (Figure 38). This can be avoided by doing a two-stage resection, initially resecting only up to the midline, and completing the resection of the 2nd vocal cord tumour about a month later (Figure 39).
/Fig%2037.png)
/Fig%2038.png)
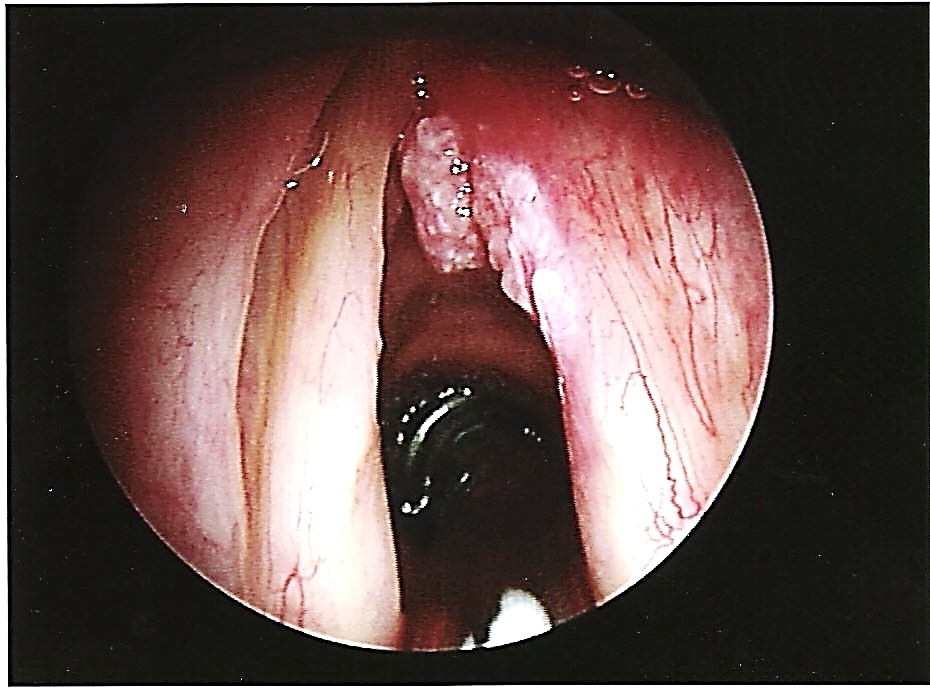
It may be difficult to correct a web. Simply dividing the web with laser invariably leads to a new web. An option is apply Mitomycin C after excising of the scar tissue with laser, or to divide the web with laser and to place a silastic keel for 3 weeks to allow the cut edges to epithelialize 3 (Figures 40, 41, 42). The keel is fixed into position with sutures that are passed through needles passed from externally above and below the anterior commissure 3. Keels can be cheaply made in the operating room either by folding cellotape back onto itself over a nylon suture (Figure 40), or by weaving a suture through the edge of a silastic sheet (Figure 41).
/Fig%2040a.png)
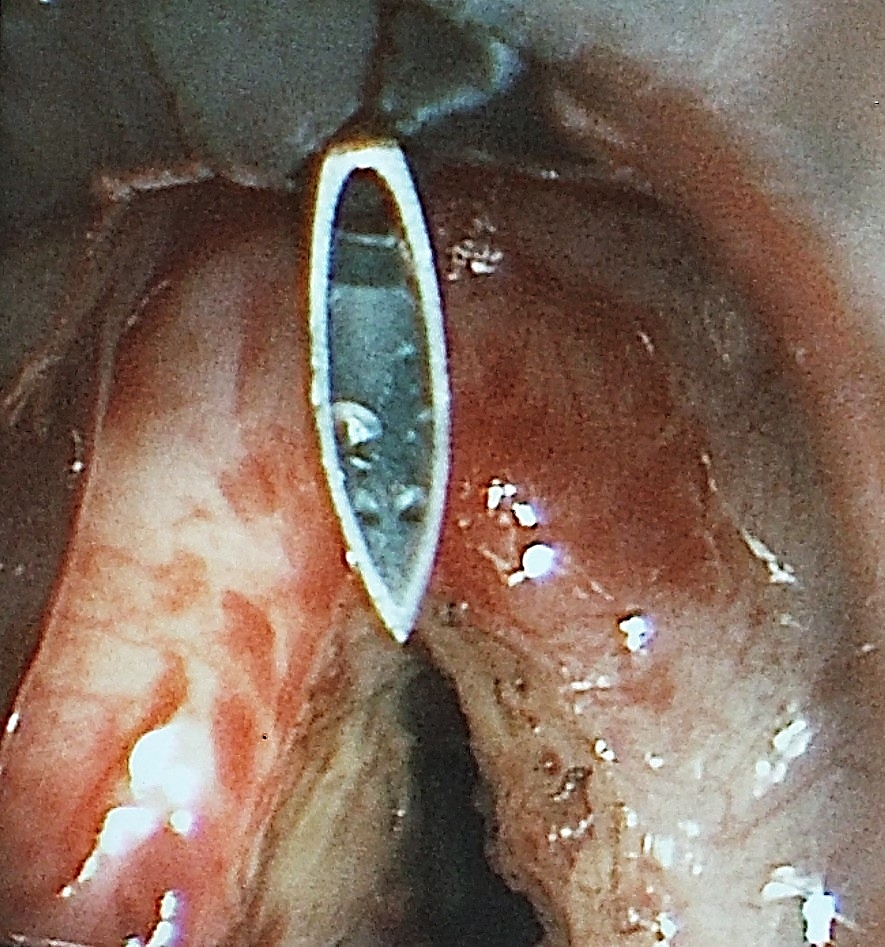
/Fig%2041a.png)
/Fig%2041b.jpg)
/Fig%2042.jpg)
An alternative which has been successful in the 1st author’s hands is to divide the anterior commissure and to temporarily lateralise the vocal cord(s). Two injection needles are passed from the outside of the neck through thyroid cartilage above and below the vocal cord. A suture is fed in and out of the larynx through the needles, and the cord is lateralised by tying the sutures together outside the neck for 3 weeks (Figure 43, 44).
/Fig%2043.png)
/Fig%2044.png)
Supraglottic carcinoma is resected using a distending laryngopharyngoscope (Figure 9), graspers, and liga clip applicators to clip the superior laryngeal vessels that traverse the pharyngoepiglottic folds. Because the vocal cords are not being operated on, the surgeon sets the laser at a higher power (>5W), slightly larger spot size, and on superpulse mode so as to both cut and coagulate. Surgery is done under high magnification, maintaining a distance of >5mm from the edge of the tumour. Provided a neck dissection is not done simultaneously, antibiotics are not generally required.
Cancer of suprahyoid epiglottis
The suprahyoid epiglottis can be resected with only transient and relatively minor difficulty with swallowing (aspiration) that settles once postoperative pain resolves.
Cancer of infrahyoid epiglottis and false vocal cords
Postoperative aspiration is minor when compared to open supraglottic laryngectomy; the nasogastric tube is usually removed within a few days. The resection is adapted to the tumour and may e.g. involve only removing one half of the epiglottis
/Fig%2045.jpg)
/Fig%2046.jpg)
Figures 47a-d & 48a-c illustrate supraglottic cancers, their excision and recovery.
/Fig%2047a.jpg)
/Fig%2047b.jpg)
/Fig%2047c.png)
/Fig%2047d.png)
/Fig%2048a.jpg)
/Fig%2048b.png)
/Fig%2048c.png)
Cancers of the piriform fossa may extend medially into paraglottic space, cricoid and cricoarytenoid joint; anteriorly into pre-epiglottic space; and laterally to invade thyroid cartilage, and behind thyroid lamina, to the soft tissues of the neck and the carotid sheath (Figure 47).
At diagnostic endoscopy assess
Resection technique
Surgery is done under high magnification through laryngoscopes and a distending laryngopharyngoscope (Figure 9) using graspers, and liga clip applicators to clip the superior laryngeal vessels that traverse the pharyngoepiglottic folds. Maintain a distance of 5-10mm from the mucosal edge of the tumour. Because the vocal folds are not being operated on, the surgeon sets the laser at a higher power (>5W), slightly larger spot size, and on CW mode so as to both cut and coagulate tissue. Reduce the power when dissecting posterior to the thyroid lamina in the region of the carotid sheath. Both authors have on occasion exposed the carotid artery during resection for hypopharyngeal cancers without any adverse effects; the vessel was either left uncovered or sealed with fibrin tissue glue. The tumour is excised in segments, taking care to orientate the specimens relevant for histology of the deep margins for the pathologist. With major resections insert a nasogastric feeding tube under direct vision to avoid the tube transgressing the tumour bed and entering the soft tissues of the neck, or arrange for a PEG. Provided a neck dissection is not done simultaneously, antibiotics are not required.
Figures 51&52 show examples of piriform fossa and postcricoid cancers both before resection and after healing have occurred.
/Fig%2051a.png)
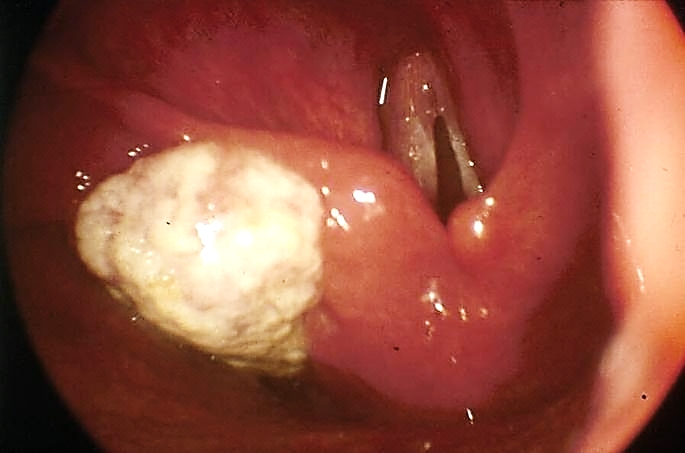
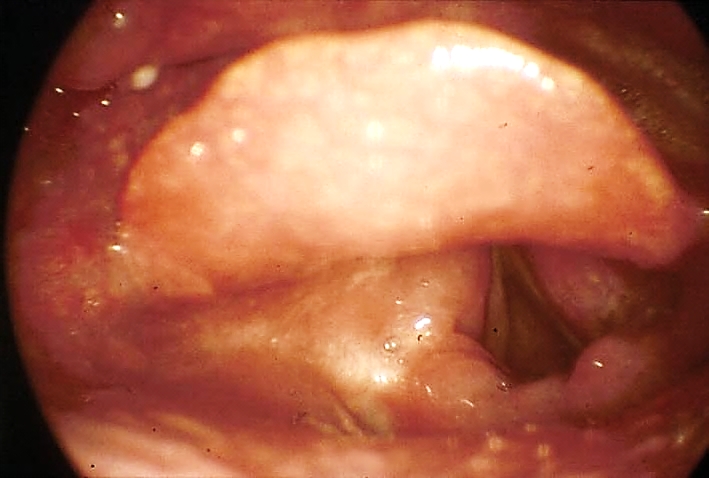
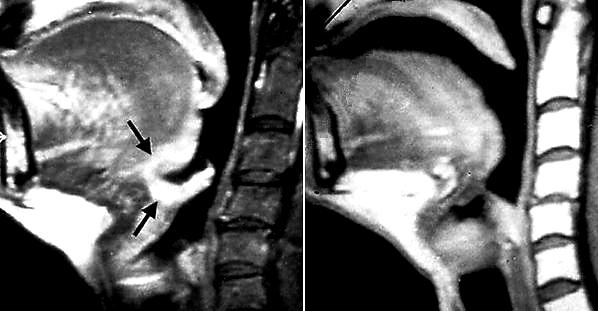
Functional outcomes are superior to external surgical resections. Depending on anatomical factors, accessing BOT tumours for laser excision may be very easy or impossible (other than in the hands of the expert using small scopes.
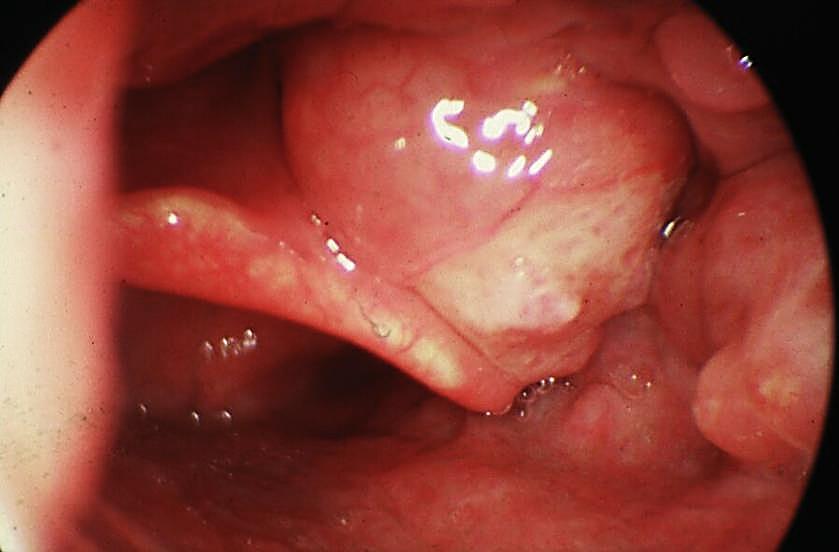
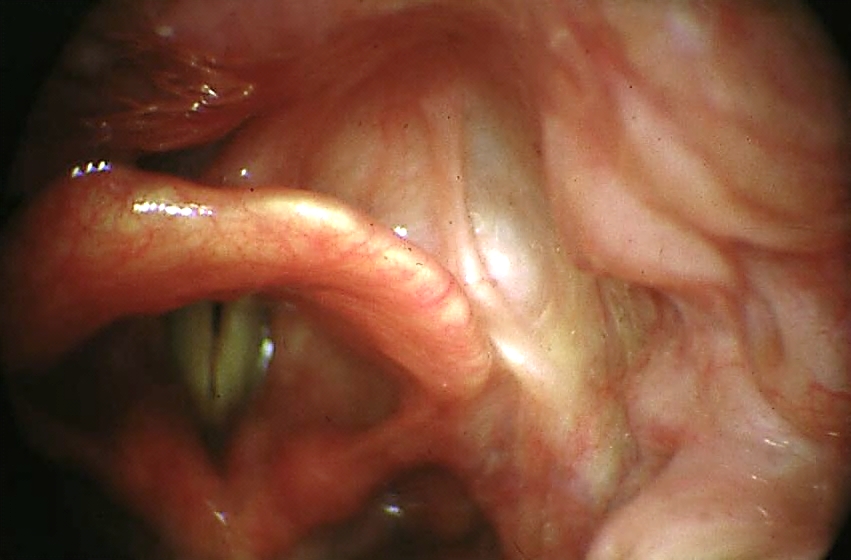
Key issues relating to BOT cancer resection:
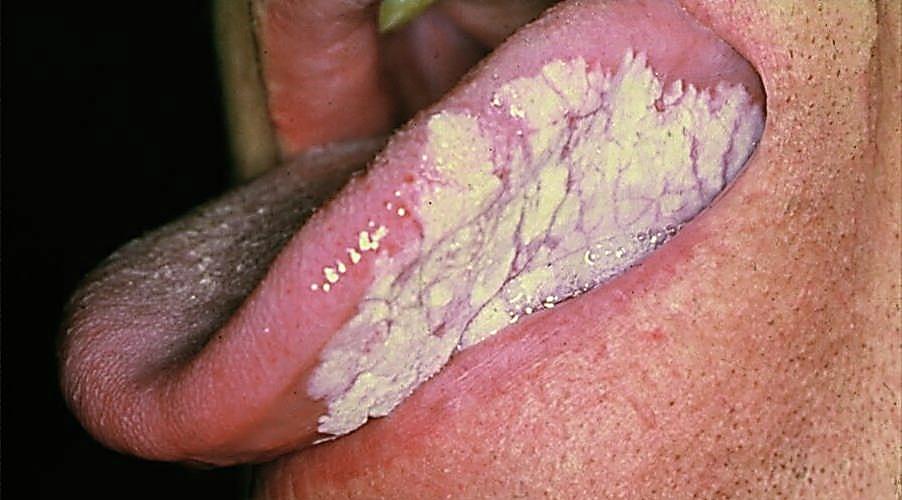
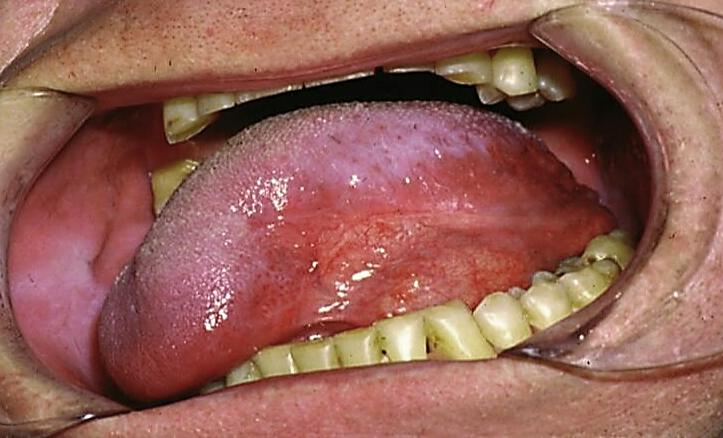
Oral and oropharyngeal lesions may be excised with CO2 laser using the micro-scope. All lesions should be excised for histological evaluation i.e. not vaporised. Figures 55a & b illustrate the excellent functional outcome after leaving a tumour bed to heal following laser excision.
Figures 56a & b illustrate resection of cancer of the soft palate.
/Fig%2056a.jpg)
/Fig%2056b.jpg)
Internal laryngocoeles and saccular cysts do very well with wide marsupialisation with CO2 laser and removing the contents of the cyst with a sucker (Figures 57, 58).
/Fig%2057.jpg)
/Fig%2057.jpg)
The objectives of surgery for viral papillo-matosis are not to eradicate all virally infected tissue, but to maintain airway and voice. The most popular techniques are CO2 laser and microdebriders. Ablation of papillomata is done with CO2 laser on a low power setting and by slightly defocusing the beam to create a larger spot size. Surgery should be conservative to preserve voice.
/Fig%2059.png)
Papillomata at the anterior commissure present a challenge in terms of avoiding webbing and a poor voice. One may elect to stage the treatment at the anterior commissure by ablating only one side at a time to avoid webbing. Tracheal papillomata can be ablated using the microscope by advancing a smaller and longer laryngoscope between the vocal cords; or by using a laser bronchoscope (Figure 3).
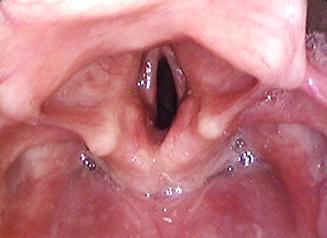
Intubation granulomas originate on the traumatised vocal process of the arytenoid (Figure 60).
/Fig%2060.jpg)
/Fig%2061.jpg)
Only if they do not resorb after a few months and remain symptomatic are they removed, taking care not to expose the cartilage so as to reduce the likelihood of recurrence (Figure 61)
With surgery done to relieve airway obstruction due to bilateral vocal fold paralysis, the patient has to be forewarned about the trade-off of airway vs. voice quality, and that a 2nd operation may be required should the airway still not be adequate. While some surgeons prefer initially to do unilateral posterior cordectomy, the 2nd author routinely does bilateral posterior cordectomy as in his experience this yields a good outcome both in terms of airway and voice.
The surgery is done around a small (5mm) endotracheal tube with the laryngoscope placed behind the tube so as to expose the posterior larynx. CO2 laser is used to excise only the vocal process of the arytenoid (posterior 1/3 of vocal fold) and a variable amount of tissue lateral to the vocal process (Figure 62). It is important that the anterior 2/3 (vibrating part) of the vocal fold be preserved to optimise voice quality.
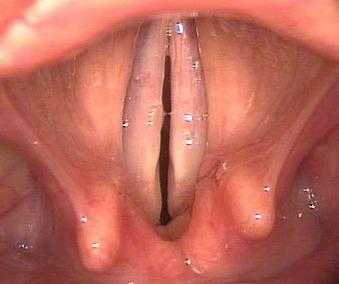
The final result is only apparent months after the surgery once fibrosis and healing have matured. The voice quality is usually very adequate as there is improved airflow through the larynx due to the larger airway, and because surgery is limited to the posterior glottis (Figure 63).
/Fig%2063.png)
CO2 laser is best suited to thin webs. The surgery is done by passing the smaller, longer laryngoscope past the vocal folds. Anaesthesia is done with an open airway, either having the patient breathing spontaneously with intravenous anaesthesia, or with intermittent jet ventilation; the 2nd author prefers using intermittent extubation with the surgery done during apnoeic intervals. The stricture is incised in a radial fashion, preserving mucosal bridges between the cuts (Figures 64).
/Fig%2064.jpg)
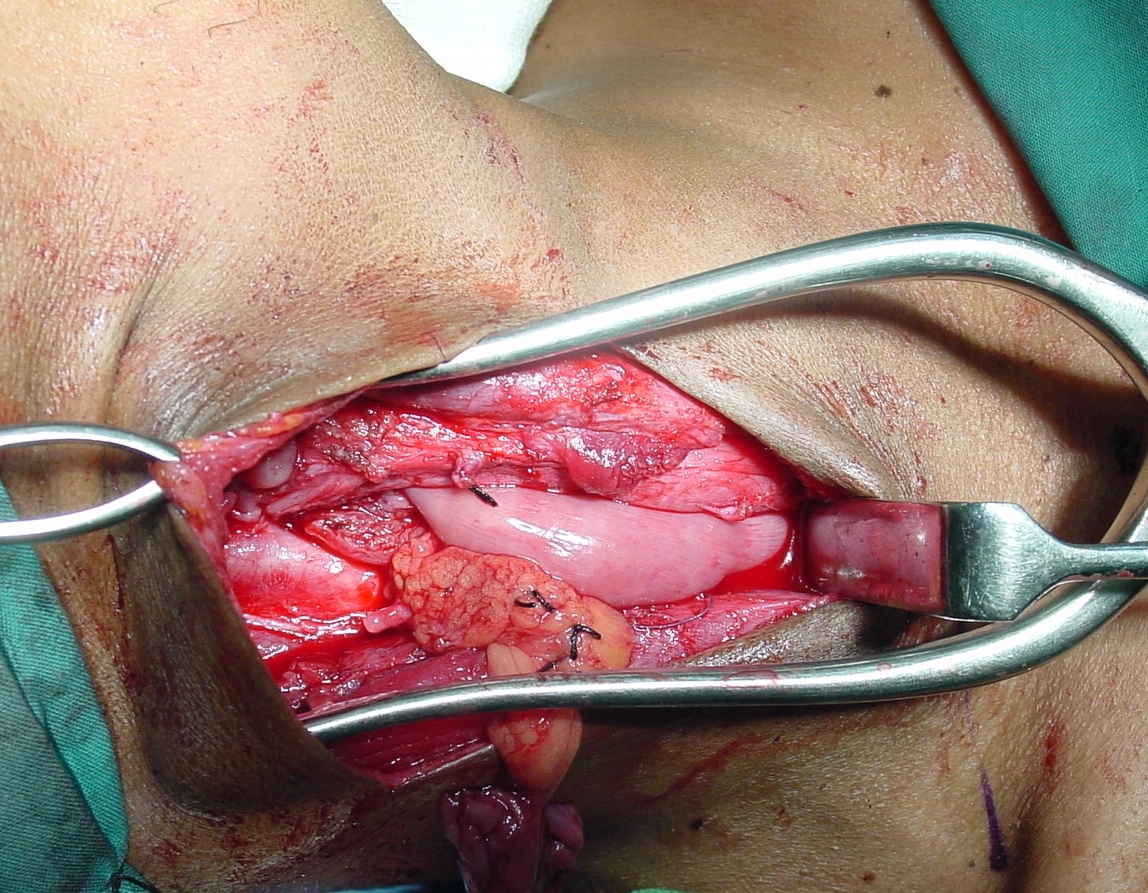
Endoscopic diverticulotomy involves dividing the “party wall” between the oesophagus and the diverticulum, as well as the cricopharyngeus muscle which is located within the superior part of the “party wall” (Figure 65). It may be performed with a stapler (Figure 66) or with CO2 laser. The 2nd author (W.S) has preferred CO2 laser endoscopic diverticulotomy for the past 30 years. Both techniques have proven to be effective and safe.
/Fig%2065.png)
/Fig%2066.jpg)
/Fig%2067.png)
CO2 laser is particularly useful for pouches of <4cms because the stapler does not divide the distal 1-2cms of the party wall due to its design (Figure 65).
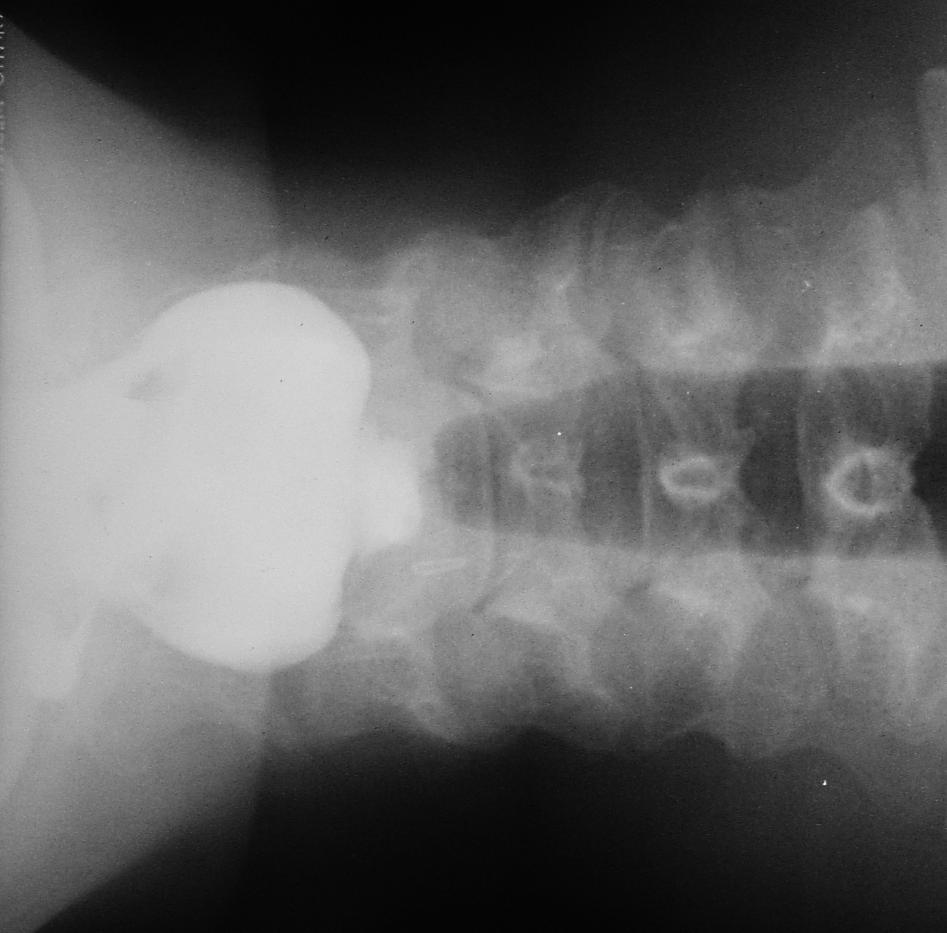
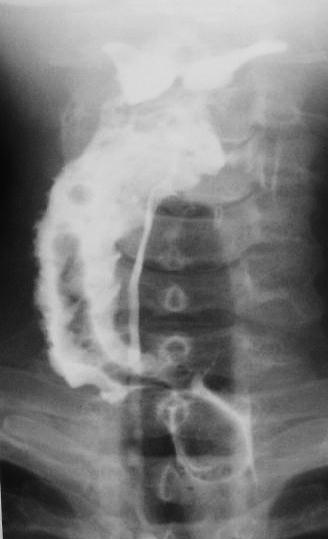
The operation is done with a Weerda diverticuloscope (Figure 68). It is not always possible to insert the scope due to anatomical limitations. In inexperienced hands the Weerda scope is a particularly dangerous instrument as it may be difficult to insert and may penetrate the posterior pharyngeal wall or the pouch causing cervical sepsis and mediastinitis (Figure 69).
/Fig%2068.png)
/Fig%2069.png)
/Fig%2070.jpg)
Patients have surprisingly little pain following laser resections. They commonly mention having to clear their throats for a few weeks due to discharge from the surgical bed.
/Fig%2071.jpg)
/Fig%2072.png)
Johan Fagan MBChB, FCORL, MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town
South Africa
johannes.fagan@uct.ac.za
Wolfgang Steiner MD, Hon. FRCS (Engl)
Professor Em. & Past Chairman
Dept of ENT, Head & Neck Surgery
University of Goettingen
Göttingen, Germany
wolfgang.p.steiner@googlemail.com