Thyroidectomy is a very common operation. The most frequent indications for surgery are uncertainty about the nature of a thyroid mass, or treatment of a large goitre causing compressive symptoms, thyroid cancer, or thyrotoxicosis refractory to medical management.
A detailed knowledge of thyroid anatomy is a prerequisite for thyroid surgery. Particular attention needs to be paid to identifying and preserving the recurrent laryngeal nerve (RLN), the external branch of the superior laryngeal nerve (SLN) and the parathyroid glands.
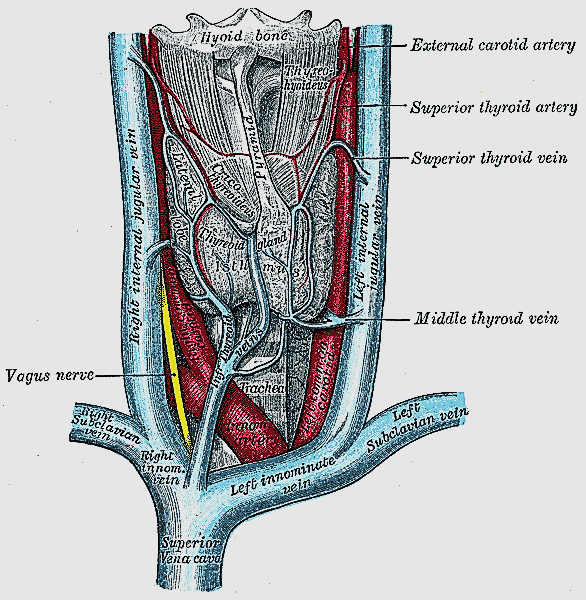
The gland consists of two lateral lobes joined anteriorly by the isthmus which typically overlies the 2nd and 3rd tracheal rings (Figures 1, 2).

The pyramidal lobe is a superior extension near the midline and is present in up to 70% of cases (Figure 1).
The thyroid is encased by a fine capsule of pretracheal fascia which is part of the
middle layer of deep cervical fascia. The fascial layers fuse to form Berry’s
ligament which posteromedially firmly attaches the thyroid to the trachea.
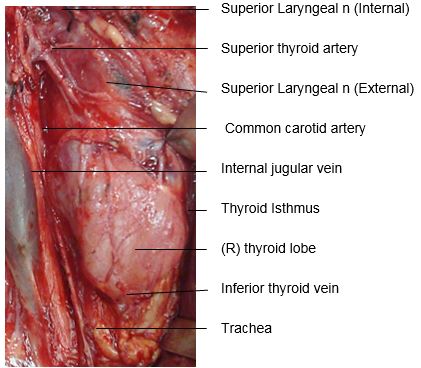
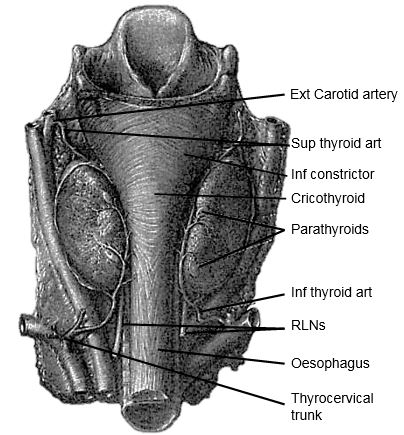
The gland encircles the anterior and lateral aspects of the cervical trachea and is applied to the surface of the larynx. Lateral to the gland are the carotid sheath (common carotid artery, internal jugular vein and vagus/Xn) and the sternocleidomastoid (SCM) muscle (Figures 1, 2). Anterior to the thyroid are the infrahyoid strap muscles (sternohyoid and sternothyroid) (Figure 3). The deep/medial anatomical relations are the thyroid (caudad to attachment of sternothyroid muscle to oblique line) and cricoid cartilages, trachea, inferior constrictor and cricothyroid muscles, oesophagus, superior and inferior thyroid arteries, and RLNs (Figures 3, 4, 5).
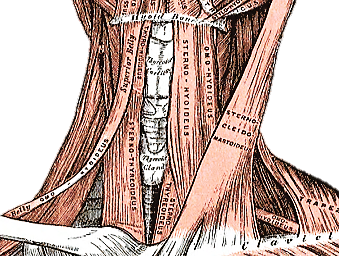
The thyroid gland weighs 15-25g. The thyroid lobes are normally cone-shaped and measure approximately 5cm in length and 2-3cms in width in both transverse and anteroposterior dimensions.
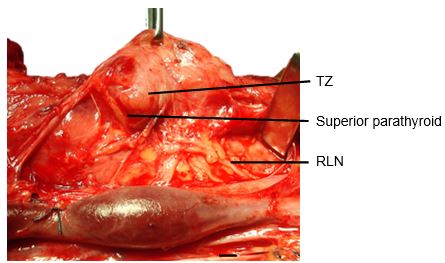
The Tubercle of Zukerkandl is a pyramidal enlargement of the lateral edge of the thyroid lobe that stems from the fusion of the lateral and medial thyroid anlages (Figure 6). It is recognisable in up to 75% of thyroids. It is anatomically closely related to the RLN, the inferior thyroid artery, Berry's ligament and the superior parathyroid gland.
The tubercle usually projects lateral to the RLN. Elevating the tubercle allows the RLN to be readily located. Less commonly the RLN courses lateral to an enlarged tubercle; this places the nerve at risk of injury. The superior parathyroid gland, also derived from the 4th branchial cleft, is commonly located close to and cephalad to the tubercle.
The arterial supply is based on the superior thyroid (STA) and inferior thyroid (ITA) arteries. Occasionally the thyroidea ima artery is encountered inferiorly but is seldom of surgical relevance. It arises from the innominate artery or aortic arch and ascends along the front of the trachea.
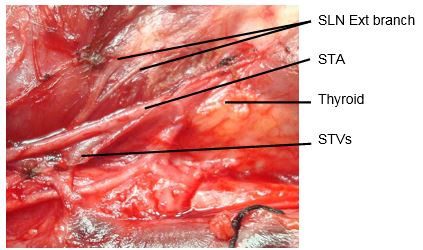
The superior thyroid artery (STA) is the first branch of the external carotid artery (Figures 2, 5, 7). It courses over the external surface of the inferior constrictor muscle of the pharynx, entering the gland posteromedially just below the highest point of the upper pole where it usually is located superficial to the external branch of the SLN (Figure 2). Its branches communicate with the ITA and cross to the contralateral thyroid lobe via the thyroid isthmus.
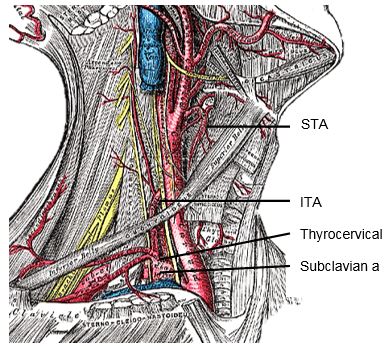
The inferior thyroid artery (ITA) is a branch of the thyrocervical trunk which originates from the subclavian artery (Figures 5, 7). It courses superiorly along the surface of the anterior scalene muscle before it turns medially behind the carotid sheath from where it reaches the inferior pole of the thyroid (Figure 5). It provides blood supply to the thyroid, upper oesophagus and trachea, and is the sole arterial supply to all the parathyroid glands, both superior and inferior. The relationship of the ITA and RLN is reviewed later.
Venous drainage is quite variable and occurs via a capsular network of thin-walled, freely intercommunicating veins which drain through the superior thyroid veins (adjacent to the STA), the inferior thyroid veins (exit the inferior pole), and the middle thyroid vein(s), which course laterally to drain directly into the internal jugular vein (Figure 1). The middle thyroid vein is surgically most relevant; it is encountered early during thyroid mobilisation, and failure to secure it causes bothersome bleeding.
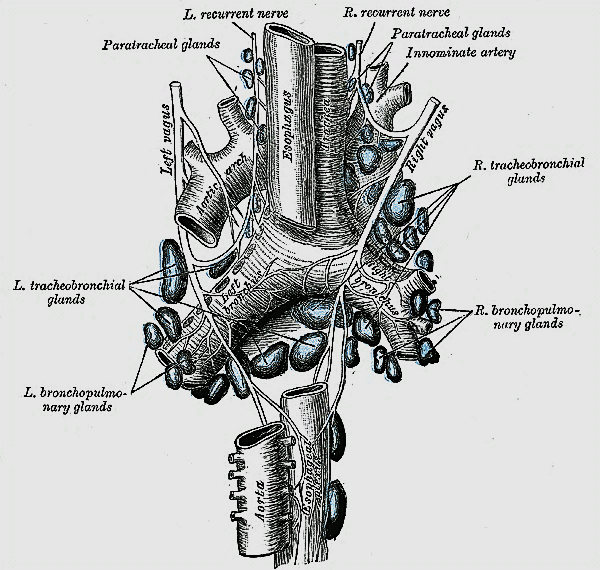
Lymphatic drainage parallels the venous drainage and occurs to the lateral deep cervical and pre- and paratracheal lymph nodes (Figure 8). Understanding the pattern of nodal drainage is particularly important in managing patients with thyroid cancer since the cervicocentral compartment is most commonly involved in metastatic thyroid cancer.
During thyroid surgery, identification and preservation of the RLN and all of its divisions is essential to minimise morbidity. The RLN innervates all the intrinsic muscles of the larynx except the cricothyroid muscle (SLN) and provides sensory innervation to the larynx. Even minor neuropraxia may cause dysphonia; irreversible injury confers permanent hoarseness. The reported incidence of RLN injury during thyroidectomy is 0 - 28% and is the most common reason for medicolegal claims following thyroidectomy.
The RLNs originate from the Xn. After circling around the subclavian artery (right) and aortic arch (left) the RLNs ascend superiorly and medially toward the tracheoesophageal groove (Figures 8, 9). The right RLN enters the root of the neck from a more lateral direction. Its course is less predictable than that of the left RLN. The RLNs enter the larynx deep to the inferior constrictor muscles and posterior to the cricothyroid joint.
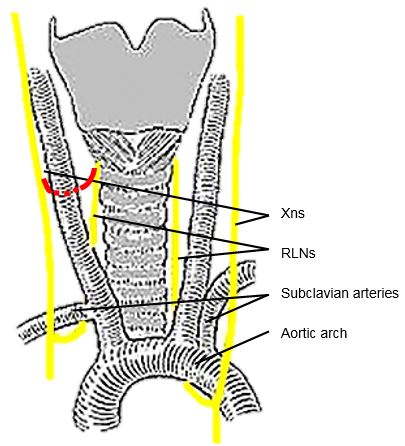
The RLN may be non-recurrent in approximately 0.6% of patients i.e. does not pass around the subclavian artery, but branches from the Xn higher in the neck, passing directly to the larynx close to the superior thyroid vessels (Figure 9). This aberration almost always occurs on the right side and is associated with a retroesophageal subclavian artery.
Knowledge of the anatomical relationships of the RLN to the tracheoesophageal groove, ligament of Berry, and ITA is essential. The course of the RLN with respect to the ITA is quite variable. Most commonly it crosses behind the branches of the artery, more predictably so on the left. However, the nerve may pass deep to, superficial to, or between the terminal branches of the ITA. Up to twenty anatomical variations have been described. In Figure 10 the RLN is seen to pass anterior to the artery.
The majority of RLNs are located within 3mm of Berry’s ligament; rarely the nerve is embedded in it, and more commonly lies laterally to it.
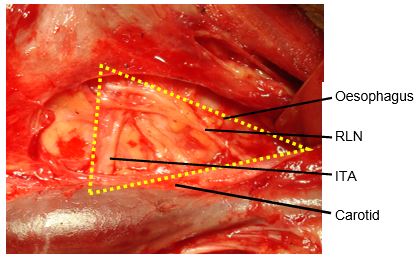
Classically, the RLN is identified intra-operatively in Simon’s triangle, which is formed by the common carotid artery laterally, the oesophagus medially, and the ITA superiorly (Figure 11).
The Tubercle of Zukerkandl may also be used as an anatomical landmark to identify the nerve (Figure 6). The RLN generally courses between this structure and the trachea. However, this relationship can vary with enlargement of the tuberculum thereby placing the nerve at risk during exploration.
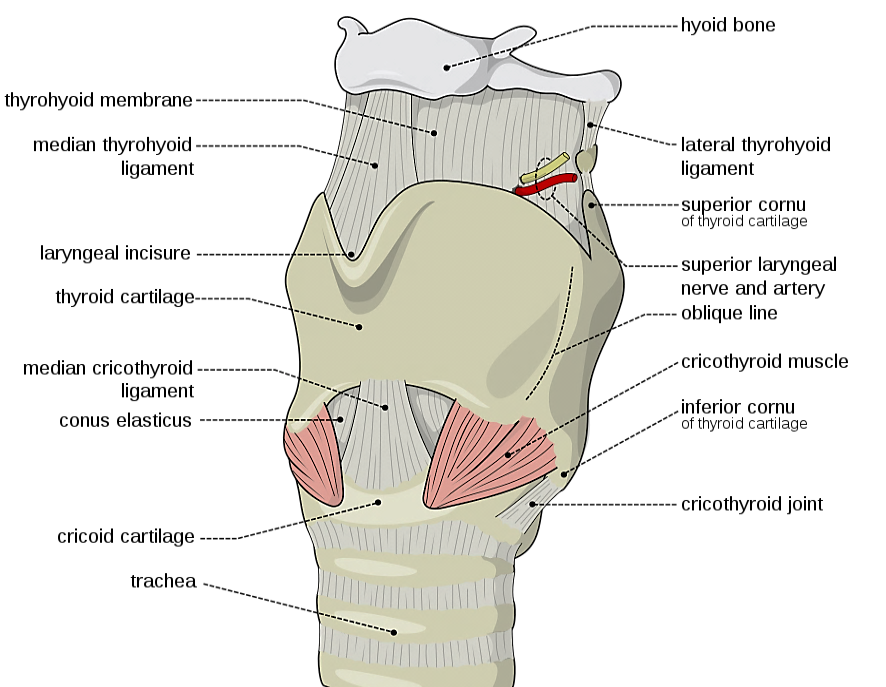
The SLN is a branch of the Xn and has both an external and internal branch (Figures 2, 12). The internal branch is situated above and outside the normal field of dissection; it is sensory and enters the larynx through the thyrohyoid membrane.
The external branch innervates the cricothyroid muscle, a tensor of the vocal cord. Injury to the SLN causes hoarseness, decreased pitch and/or volume, and voice fatigue. These voice changes are more subtle than those relating to a RLN injury, and are frequently underestimated and not reported. The external branch of the SLN is at risk because of its close proximity to the STA (Figures 12, 13). Understanding its relationship to the upper pole of the thyroid and the STA is crucial to preserving its integrity.
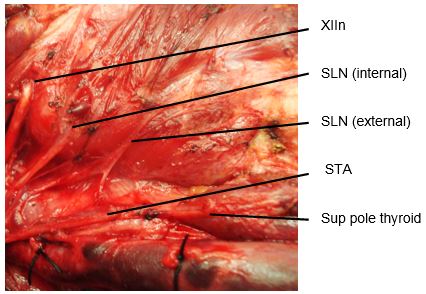
The usual configuration is that the nerve is located behind the STA, proximal to its entry into the superior pole of the thyroid. The relationships of the nerve to the superior pole and STA are however extremely variable. Variations include the nerve passing between the branches of the STA as it enters the superior pole of the thyroid gland; in such cases it is particularly vulnerable to injury.
There are typically four parathyroid glands; however, supernumerary glands have been reported. The parathyroid glands are generally symmetrically located in the neck. Their characteristic golden colour varies from yellow to reddish brown, and permits them to be distinguished from the pale-yellow colour of adjacent lymph nodes, thymus, mediastinal fat, and the dark red thyroid parenchyma. They are usually oval and measure 3–8 mm. The ITA is the predominant vascular supply to both the upper and lower parathyroids. Consequently dividing the trunk of the ITA is discouraged as it places all the parathyroids on that side at risk of ischaemic injury.
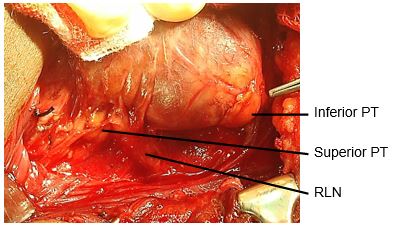
The superior parathyroid glands originate from the 4th pharyngeal pouch and attach to the posterior surface of the caudally migrating thyroid. They have a much shorter migration distance compared to the inferior parathyroid glands; this accounts for their more predictable location. They are embryologically and anatomically closely related to the Tubercle of Zuckerkandl, and are usually located at the level of the upper two-thirds of the thyroid, in a posterior position, about 1cm above the crossing point of the RLN and inferior thyroid artery (Figure 6). Ectopic positions of the superior parathyroid glands such as in the posterior neck, retropharyngeal and retroesophageal spaces and intrathyroidally are very uncommon (1%).
The dorsal wing of the 3rd pharyngeal pouch gives rise to the inferior parathyroid glands. They join the thymus as it travels caudally and medially to its final position in the mediastinum. This accounts for the fact that they are usually found in a plane ventral to that of the superior parathyroid glands, and that ectopic inferior parathyroid glands can be found anywhere along this large area of descent up to the superior border of the pericardium. Their commonest location is between the lower pole of the thyroid and isthmus, equally commonly on the anterior or the posterolateral surfaces of the lower pole of the thyroid (42%, Wang et al); or in the lower neck in proximity to the thymus (39%). Other locations are: lateral to the thyroid or within the carotid sheath (15%), within the mediastinal thymic tissue and the pericardium (2%).
If the RLN’s course is viewed in a coronal plane, then the superior parathyroid gland is located deep (dorsal) and the inferior parathyroid superficial (ventral) to the plane of the nerve (Figures 14a, b).
Thyroid lobectomy: Either lobe is removed, usually with a small segment of the thyroid isthmus; the contralateral lobe is left undisturbed. It is most commonly performed as a diagnostic procedure for a thyroid nodule of uncertain nature. It may be a sufficient for cure in some cases of thyroid carcinoma with favourable prognostic criteria.
Subtotal thyroidectomy: 90-95% of thyroid tissue is removed bilaterally, leaving a small (1x2cm) thyroid remnant in situ overlying the RLN. This operation has slowly lost favour as it is by its very nature inexact, is prone to recurrence of the thyroid pathology, and in expert hands does not result in lower rates of RLN injury when compared to total thyroidectomy.
Total thyroidectomy: Both right and left lobes, isthmus and pyramidal lobe (when present) are removed; no macroscopic thyroid tissue is left in situ. This is the procedure of choice for the treatment of thyroid carcinoma and is commonly performed for a MNG with compressive symptoms, or for thyrotoxicosis.
Bilateral RLN injury causing airway compromise and hypoparathyroidism causing hypocalcaemia in situations where monitoring serum calcium and treating hypo-calcaemia with calcium and Vitamin D are not possible may have fatal consequences. Regardless of surgical expertise, the complication rates rise with the extent of resection. Unilateral thyroid lobectomy rarely causes RLN injury and almost never causes significant hypoparathyroidism. Subtotal thyroidectomy preserves the blood supply to the ipsilateral parathyroid glands and reduces the risk of hypo-calcaemia. Total thyroidectomy is however associated with both increased short- and long-term morbidity relating to RLN paralysis and hypocalcaemia, particularly in an occasional thyroid surgeon’s hands. Short-term complication rates for total thyroidectomy occur in 10-40% of patients; long-term complications (mainly hypoparathyroidism) occur in 5-20%. Most thyroidectomies are done in general hospitals by surgeons not specialising in endocrine surgery; complication rates have been reported to correlate with the number of thyroidectomies done. In the absence of convincing evidence that total thyroidectomy confers survival benefit in favourable differentiated thyroid cancers (especially when I131 therapy is not available), coupled with the morbidity and mortality of total thyroidectomy, the occasional thyroid surgeon or the surgeon practising in a setting where calcium monitoring and replacement are suboptimal may therefore elect rather to perform thyroid lobectomy or subtotal thyroidectomy for differentiated thyroid cancer.
Ultrasonography (US) permits accurate distinction between the common thyroid pathologies and is the imaging technique of choice for a thyroid mass. Neoplasms typically cause focal enlargement within a normal gland (“solitary nodule”). Features strongly suggestive of thyroid carcinoma are hypoechogenicity, increased and haphazard vascularity patterns within the lesion, microcalcifications, irregular margins, elevated height-to-width ratio, and regional lymphadenopathy. A multinodular goitre (MNG) typically shows multiple hyper- or isoechoic nodules, some cystic changes and coarse macrocalcifications involving both thyroid lobes.
Focal thyroid masses or suspicious lymph-adenopathy should be investigated by fine needle aspiration cytology.
All patients with thyroid complaints must undergo thyroid function tests as clinical manifestations of thyrotoxicosis or hypothyroidism are notoriously unreliable. Thyrotoxicosis must first be controlled medically before surgical intervention. Failure to do so may precipitate a thyroid storm.
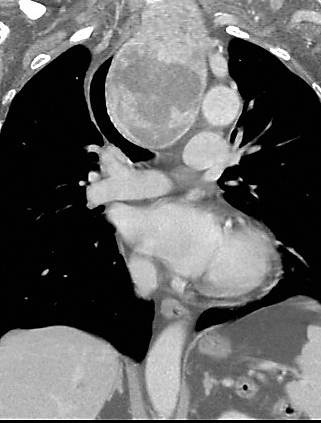
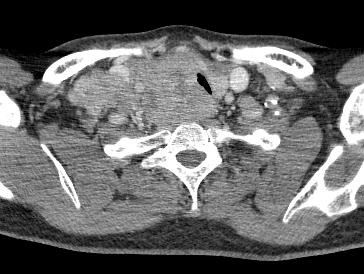
CT scans are helpful in selected cases, particularly with a MNG with a suspected retrosternal component (Figure 15), or when uncertainty exists about the extent of tracheal compression (Figure 16).
Thyroid uptake scans may be requested in cases of thyroid enlargement with thyro-toxicosis, but are not routinely done as they seldom add more information to that available from the US.
Laryngoscopy: It is medico-legally prudent to document vocal cord function prior to thyroid surgery; it is essential in patients with symptoms of dysphonia.
Scar: The incision is generally well concealed if made within a natural skin crease, but tends to descend with ageing.
Airway obstruction/wound haematoma: 1% of thyroidectomy patients develop stridor postoperatively, either due to airway oedema or a haematoma.
Voice changes: It is essential for the patient to have a clear understanding of the risk of postoperative voice change. While most are subtle and recover fully, approximately 1% of patients will have permanent hoarseness. The risk is highest for patients having surgery for carcinoma, large retro-sternal goitres, and with repeat surgery.
Hypoparathyroidism: Transient postoperative hypocalcaemia occurs in about 20% of total thyroidectomy patients. Permanent hypocalcaemia occurs following 1-5% of total thyroidectomies.
Hypothyroidism: Hypothyroidism occurs uncommonly (5%) with thyroid lobectomy. It is common practice to routinely check TSH levels approximately 6-8 weeks after surgery to identify such cases before it manifests clinically. It is self-evident that a patient will be hypothyroid following total thyroidectomy. The clinical effects only become apparent once the pre-existing thyroid hormone levels drop; this generally becomes evident 3-4 weeks following surgery. Thyroxine replacement therapy is routinely instituted immediately postoperatively to prevent hypothyroidism. The exception is if total thyroidectomy has been performed for a well-differentiated carcinoma and I131 therapy is envisaged; a hypothyroid state is deliberately induced in such patients until the I131 therapy has been administered.
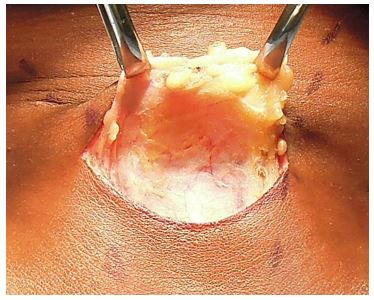
Skin incision (Figure 17): A curvilinear incision is placed in a skin crease two fingerbreadths above the sternal notch between the medial borders of the sternocleidomastoid muscles.

Placing the incision too low causes an unsightly low scar over the heads of the clavicles when the extended neck is returned to its normal position. The width of the incision may need to be extended for large goitres or for a lateral lymph node dissection.
Subplatysmal flaps: Subcutaneous fat and platysma are divided, and a subplatysmal dissection plane is developed superiorly (platysma is often absent in the midline) remaining superficial to the anterior jugular veins, up to the level of the thyroid cartilage above, and the sternal notch below (Figure 18).
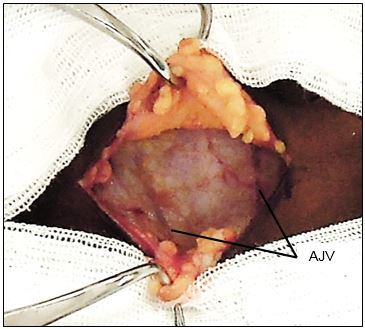
The skin flaps are secured to a fixed retractor (e.g. Jowell’s retractor) to expose the thyroid region for the remainder of operation (Figure 19).
Separating strap muscles and exposing the anterior surface of thyroid: The fascia between the sternohyoid and sternothyroid muscles is divided along the midline with diathermy or scissors (Figure 20). This is an avascular plane, though care must be taken not to injure small veins occasionally crossing between the anterior jugular veins, particularly inferiorly. The infra-hyoid (sternohyoid, sternothyroid and omohyoid) strap muscles are retracted laterally with a right-angled retractor. With massive goitres the strap muscles may be divided to improve access.
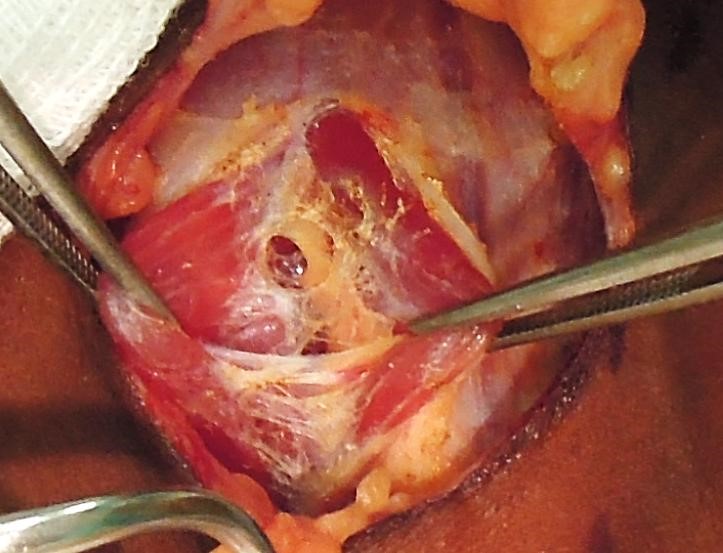
It is usual at this stage for the surgeon to move to the side of the table opposite to the thyroid lobe to be resected.
Medially rotating the thyroid: Using gentle digital retraction the surgeon rotates the thyroid gland medially (Figure 21).
Dividing the middle thyroid vein(s) (Figure 21): The first important vascular structure to come into view is the middle thyroid vein(s), which is tightly stretched by medial rotation of the gland. It is divided between haemostats and ligated with a 3/0 tie. This permits further mobilisation of the gland and delivery of the bulk of the lobe into the wound.
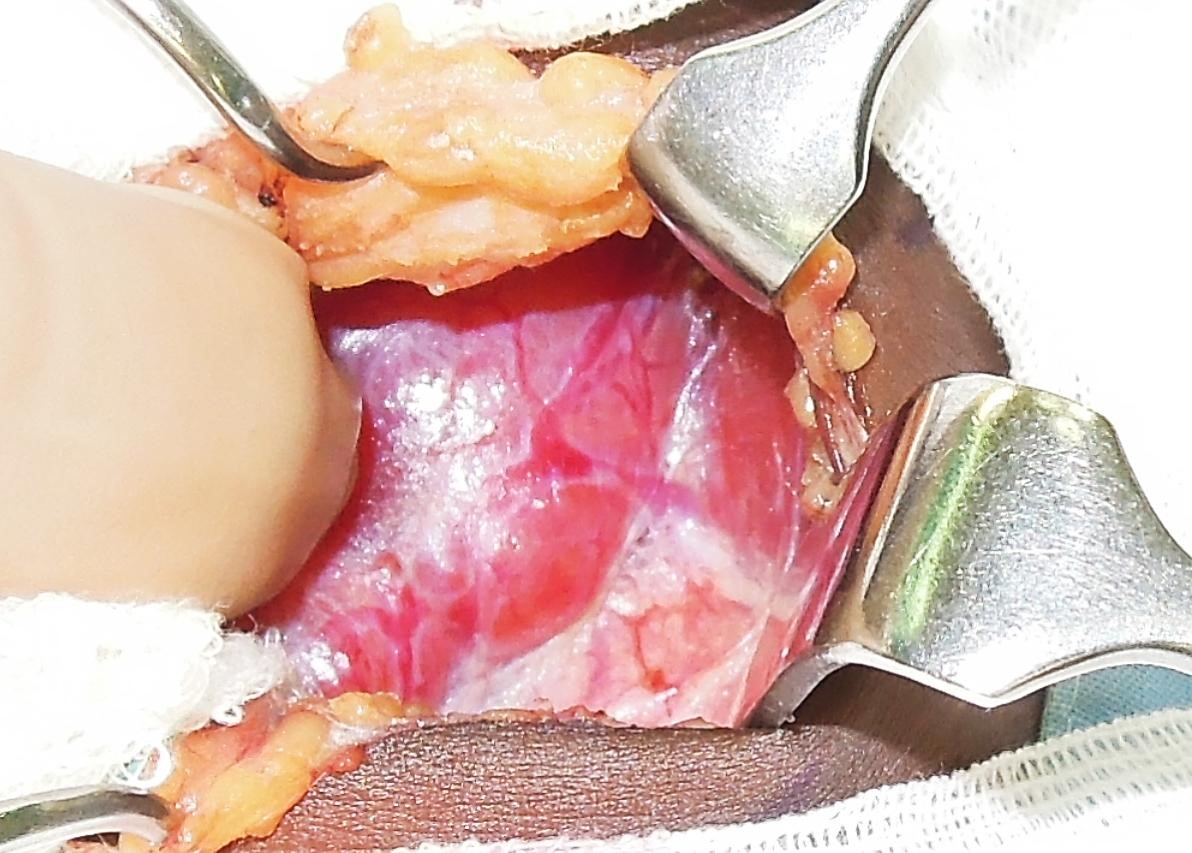
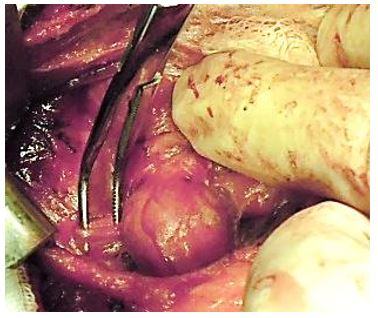
Dividing the STA (Figure 23): The retractors are repositioned to allow full visualisation of the superior pole of the thyroid. This brings the STA into view. The author does not routinely identify the external branch of the SLN, but simply takes great care to divide the artery as close to the thyroid parenchyma as possible so as to avoid injury to nerve. The superior arterial pedicle is double ligated with 2/0 or 3/0 tie.
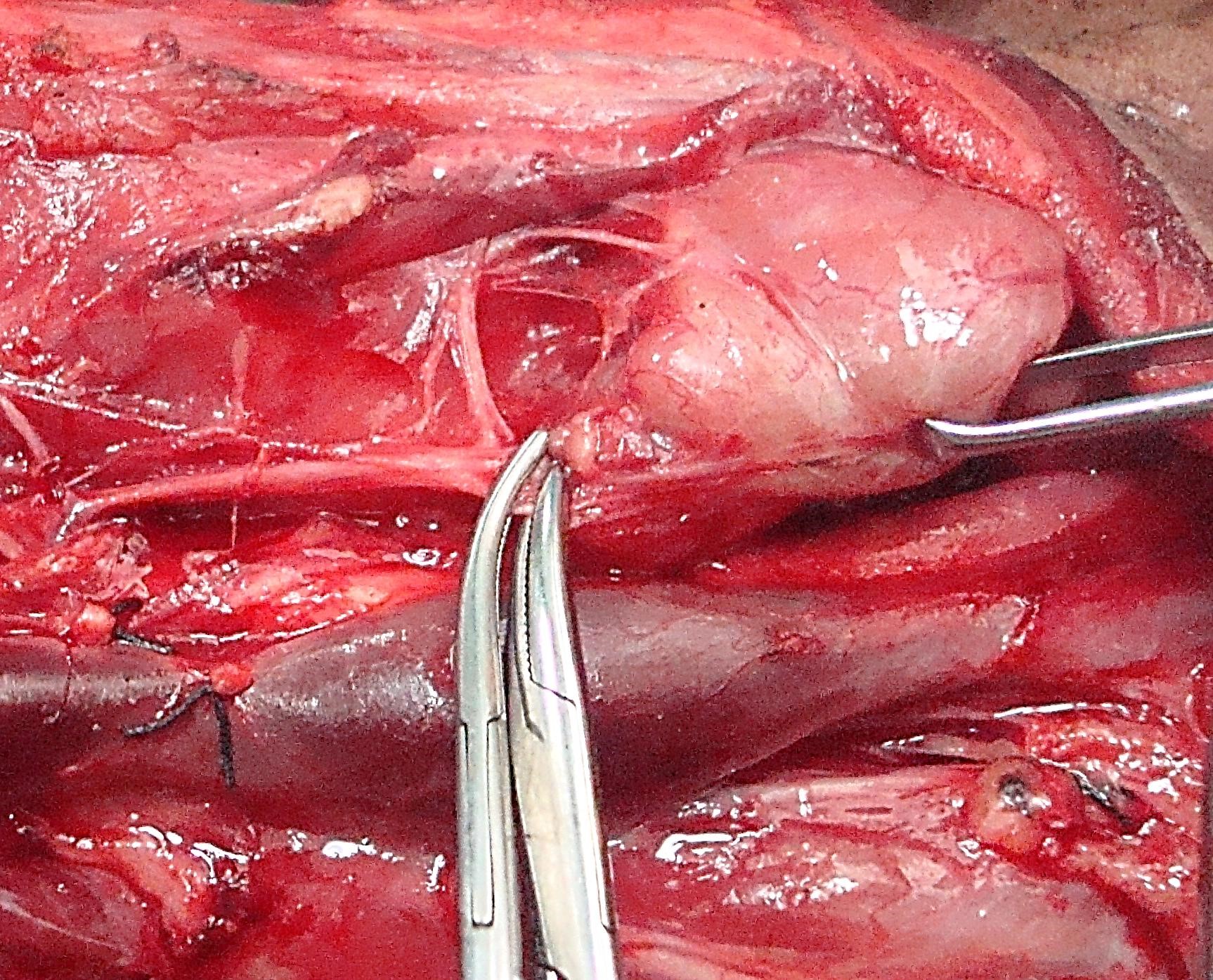
Identifying superior parathyroid gland (Figure 24, 25): Full mobilization and anterior delivery of the superior pole of the thyroid brings the region of the superior parathyroid gland into direct view. The superior parathyroid gland is normally located at the level of the upper two-thirds of the thyroid, in a posterior position and closely related to the Tubercle of Zucker-kandl, and about 1 cm above the crossing point of the recurrent laryngeal nerve and inferior thyroid artery.
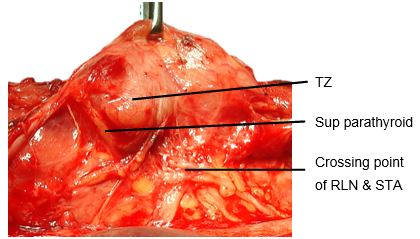
If the RLN’s course is viewed in a coronal plane, then the superior parathyroid gland lies deep (dorsal) to the plane of the nerve (Figures 14a, b). It has a characteristic rich orange/yellow colour (Figure 25). The (occasional) parathyroid surgeon may find the parathyroids difficult to identify especially if there has been bleeding in the surgical field, so care must be taken to ensure meticulous haemostasis. The gland must remain in situ with blood supply intact. This is best achieved by carefully dissecting it off the posterior aspect of the thyroid gland, and using short bursts of bipolar cautery to control bleeding.
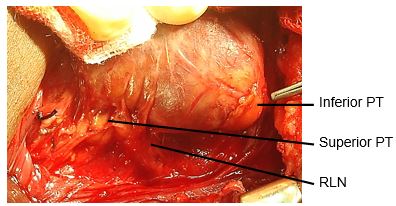
Dividing inferior thyroid veins (Figure 26): The retractors are again repositioned to expose the lower neck and the inferior thyroid vein(s). The veins are divided, and ligated. This exposes the trachea and permits full delivery of the thyroid gland outside the wound.
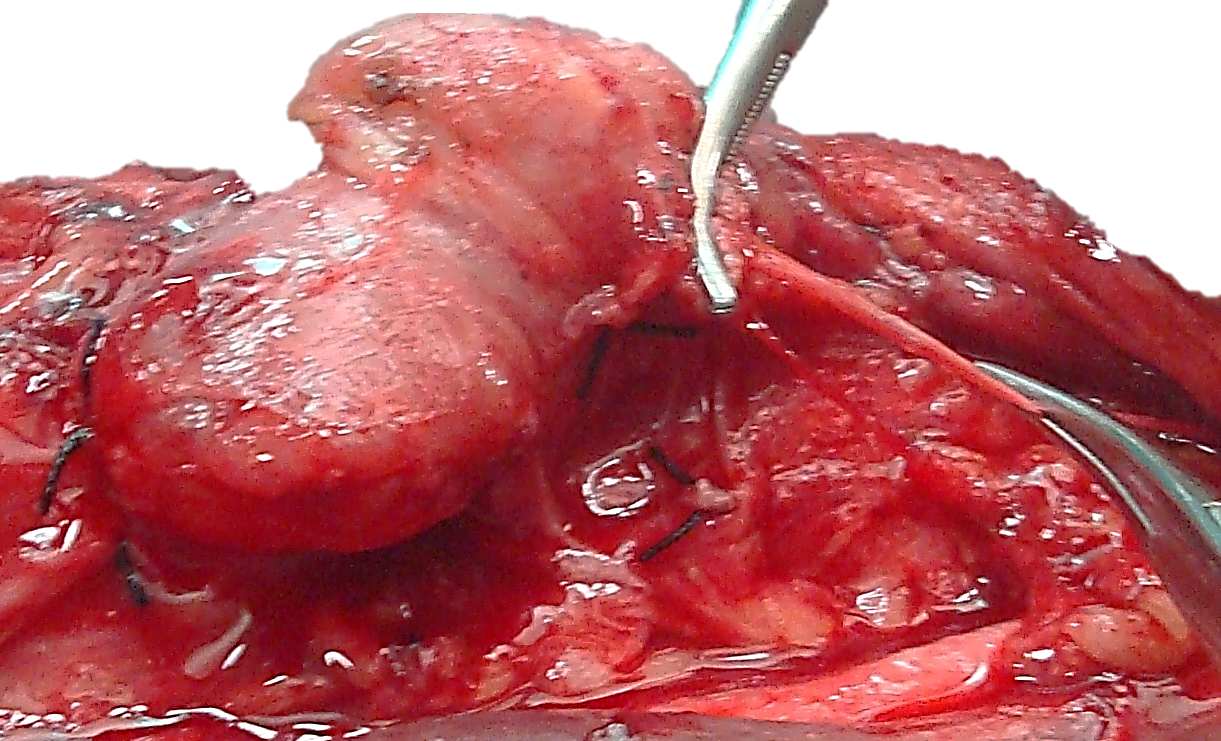
Identifying inferior parathyroid gland: The inferior parathyroid glands are normally located between the lower pole of the thyroid and the isthmus, most commonly on the anterior or the posterolateral surface of the lower pole of the thyroid (42%, Wang et al), or located in the lower neck in proximity to the thymus (39%).
If the RLN’s course is viewed in a coronal plane then the inferior parathyroid is superficial (ventral) to the plane of the nerve (Figures 14a, b). The inferior gland may now become visible on the inferior aspect of the lower pole of the thyroid or within the thyrothymic ligament (Figure 25). Care must be taken to preserve it in situ and to avoid damaging its ITA blood supply.
Identifying the RLN: The thyroid is rotated medially; lateral retraction is applied to the carotid artery and jugular vein. The RLN is located by carefully dissecting/teasing apart the tissues in Simon’s triangle which is formed by the common carotid artery laterally, the oesophagus medially, and the inferior thyroid artery superiorly (Figure 11). Others favour finding the nerve at its point of entry into the larynx approx. 0.5cm caudad to the inferior cornu of the thyroid cartilage. The nerve must remain undisturbed and in situ i.e. is not skeletonised or handled.
Pericapsular dissection of branches of ITA: It is best to individually dividing and ligating (3/0 ties) all the branches of the ITA at the capsule of the thyroid so as to reduce the risk of handling the RLN. Avoid all forms of cautery to avoid thermal injury to the nerve.
Dividing Ligament of Berry (Figure 27): The posteromedial aspect of the thyroid gland is attached to the side of the cricoid cartilage and to the 1st and 2nd tracheal rings by the posterior suspensory ligament/ Ligament of Berry. The RLN is in close proximity (<3mm) to the ligament and usually passes posterior to the ligament and must be identified before the ligament is divided with sharp dissection to free the thyroid from trachea. Carefully dissect thyroid tissue from the trachea in the region of Berry’s ligament.
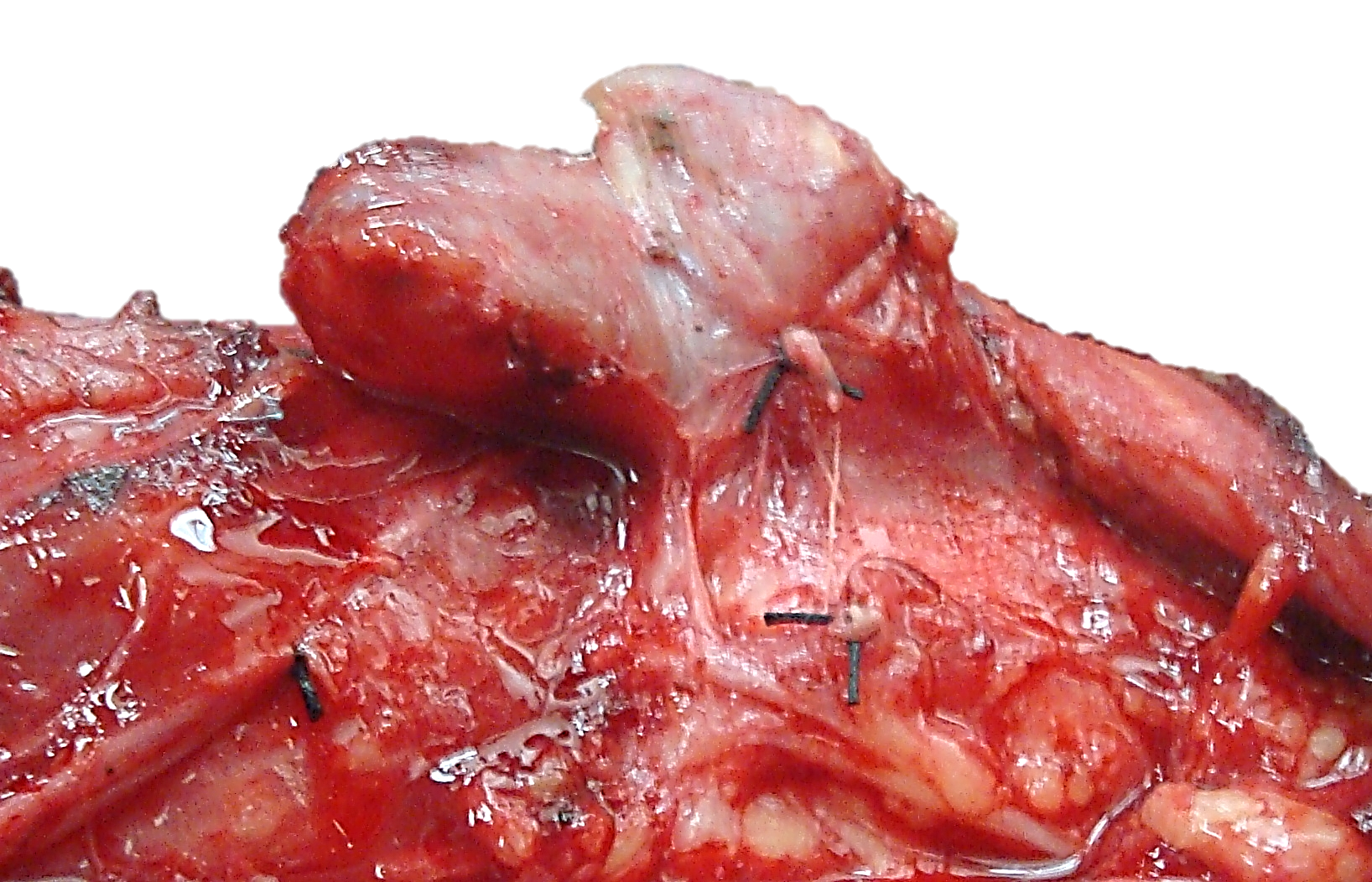
Dividing the thyroid isthmus: When doing a thyroid lobectomy the isthmus is cross-clamped with a haemostat and divided. The residual remnant is oversewn using a continuous, interlocking technique (Figure 28).
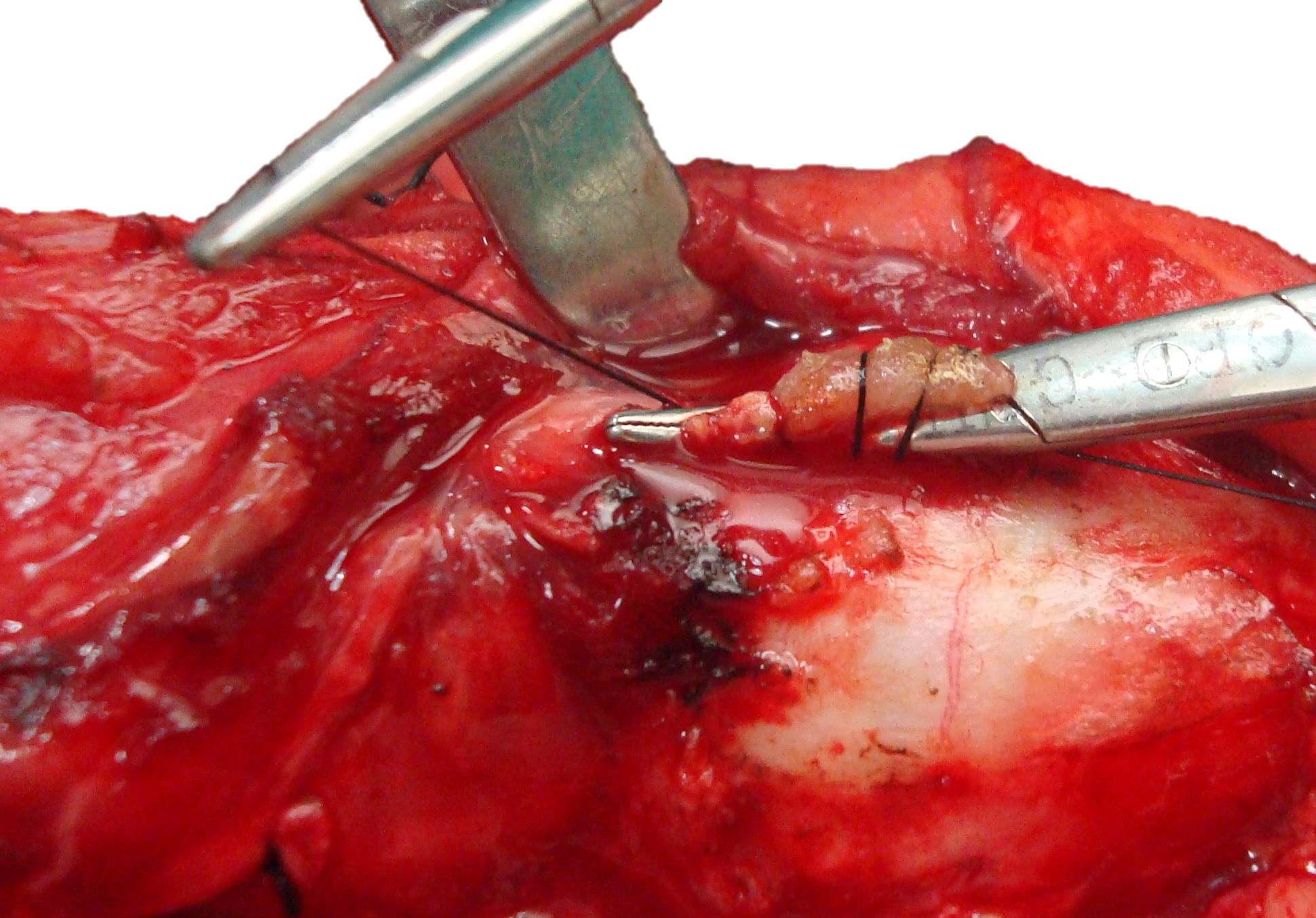
With total thyroidectomy the above surgical steps are simply repeated on the opposite side.
Wound closure
Devascularised parathyroid: Should a parathyroid gland accidentally have been devascularized or come free during dissection it should be reimplanted. This is particularly important when performing total thyroidectomy. It is stored in saline until the conclusion of the thyroidectomy, then cut into 1 mm cubes and placed in small pockets within the sternocleido-mastoid muscle.
Retrosternal goiter: Presentation, workup and technique: Retrosternal goiters may present with airway compression, stridor, and effort intolerance; venous congestion of the head and neck region is not uncommon. A CT scan is indicated as US is not ideal for the evaluation of mediastinal pathology. It is essential to exclude other causes of a mediastinal mass such as lymphoma, thymoma or teratoma. Additional steps required for removal of a large retrosternal goiter include:
Postoperative stridor: Early stridor may be encountered due to a haematoma and/or airway oedema; more uncommonly it occurs due to bilateral RLN injury or tracheomalacia. Delayed onset may be due to hypocalcaemia (tetany).
Haematoma: Avoid large, bulky dressings so as not to conceal a haematoma. A large haematoma is a surgical emergency as it may cause airway obstruction.
Seroma: Small seromas are very common and are simply followed clinically and allowed to resorb. Larger, symptomatic seromas may be (repeatedly) aspirated under sterile conditions.
RLN injury: Unilateral RLN paralysis presents as a breathy voice and hoarseness, and less commonly as dysphagia and aspiration. It may not be immediately apparent depending on the resting position of the vocal fold. Bilateral RLN paralysis usually manifests immediately following extubation with stridor or airway obstructtion. Should the patient be unable to maintain an adequate airway then emergency tracheostomy or cricothyroidotomy is indicated. Subsequent management depends on the surgeon’s knowledge of whether the RLNs were seen to be intact and hence the likelihood of vocal fold function to recover. Options might include a watchful waiting approach for up to a year or CO2 laser cordotomy/arytenoidectomy.
Continuous electrophysiologic monitoring of the RLN during thyroid surgery: Recent studies have shown that intra-operative monitoring can assist with finding the RLN, but some pitfalls limit its usefulness: there is no consensus about which types of electrodes should be used for EMG registration which is the best method for recording nerve action, or which EMG parameters should be selected as predictive of postoperative vocal cord dysfunction. The technology is not widely available, and most endocrine surgeons achieve equivalent RLN morbidity rates without it.
Tracheomalacia: This is characterized by flaccidity of the tracheal cartilages which in turn causes tracheal wall collapse. It is thought that a longstanding goiter can act as an external support structure for the trachea and predispose to secondary trach-eomalacia. Thyroidectomy unmasks trach-eomalacia causing respiratory obstruction. In clinical practice this is an uncommon cause of airway obstruction after thyroidectomy.
Thyroid specific haemostatic devices (Figure 29): The last decade has seen the introduction of thyroid specific haemostatic devices (Ultrasonic scissors/ Harmonic Scalpel and Ligasure Device), which achieve safe haemostasis and avoid the need for multiple ligatures (Figure 29).
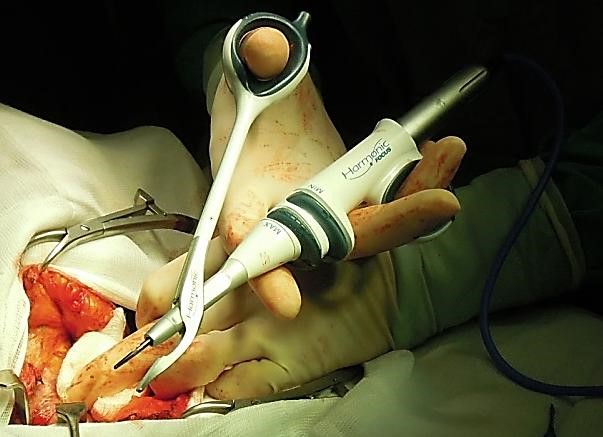
A number of randomised trials have shown equivalence between the commercially available products, and a significant reduction in operating time without an increase in complications when compared to standard thyroidectomy technique. The author uses the Harmonic Scalpel as a means of sealing and transecting vessels and to reduce surgical operating time.
Minimally Invasive Thyroid Surgery: A number of techniques have evolved in an attempt to reduce the extent of skin incisions and bring the putative benefits of minimally invasive techniques to thyroid surgery. Minimally invasive thyroidectomy can be performed via a limited 2-3cm neck incision with the visual assistance of an endoscope, specially designed retractors and a harmonic scalpel. An alternative approach is to place incisions for 3-4 ports in the axilla and periareolar regions to avoid a neck scar altogether. The clinical benefits are marginal at best, but they will continue to be driven by patient demand and the industry. Only patients with small thyroid nodules are suitable for such a surgical approach.
Surgery for Intrathoracic (retrosternal) goitres
Parathyroidectomy
Eugenio Panieri MBChB, FCS
Head: Oncology / Endocrine Surgery Unit
Associate Professor
Division of General Surgery
University of Cape Town
Cape Town, South Africa
eugenio.panieri@uct.ac.za
Johan Fagan MBChB, FCORL, MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town, South Africa
johannes.fagan@uct.ac.za