This chapter provides an overview of management of Zenker’s divertula (ZD), including detailed surgical technique, with an emphasis on effective therapy and management.
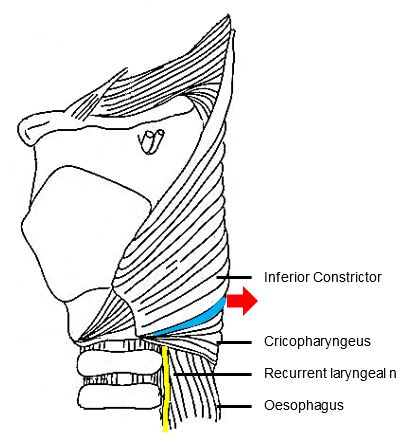
Zenker, a German pathologist, first reported 27 hypopharyngeal pulsion diverticula in 1878.1 In 1907, Killian, described the triangular dehiscence between the cricopharyngeus and inferior constrictor muscles that is the anatomic site of formation of Zenker’s Diverticula.2 (Figure 1)

Although there is a role for open surgery, management has evolved to effective, safe endoscopic techniques with proven low morbidity.
The aetiology has been debated for more than a century. Historically, authors have theorised that trauma, congenital upper oesophageal strictures, thyroid goiter, or foreign bodies may play role.3 Gastro-esophageal reflux, cricopharyngeal achalasia, persistently elevated resting tone of the cricopharyngeus, structural abnormality of the cricopharyngeus muscle, and central or peripheral neurologic disease have also been proposed as contributing or causal factors, though convincing evidence is lacking.3
However, it was Zenker who correctly described it as a pulsion diverticulum, and put earlier theories to rest.1 Since that time most authors agree that anatomic predis-position plays a central role in the patho-genesis, though the exact aetiology is still a subject of debate.
It is now widely accepted that dysfunction of the cricopharyngeus muscle plays a central aetiological role. High intraluminal pressures at the level of Killian’s triangle result in herniation of oesophageal mucosa and submucosa through this area of inherent muscular weakness. Some advocate that high intraluminal pressures produced by incoordination of the inferior constrictor muscle against a closed cricopharyngeus is responsible, while others state that high pressures are a result of the anatomic relationship between the relatively wide hypopharynx and a narrower oesophageal inlet through which food boluses pass.5 Killian’s triangle itself is of variable size, and patients with larger areas of inherent muscular weakness are likely more predisposed to a ZD.
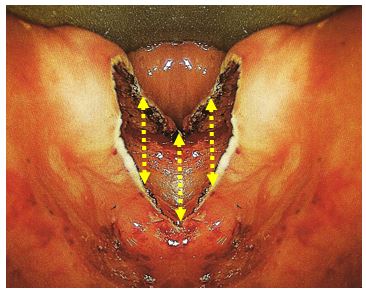
ZD is a pulsion diverticulum or herniation of oesophageal mucosa and submucosa through Killian’s triangle, which is immediately proximal to the cricopharyngeus muscle (Figures 1, 2, 3).
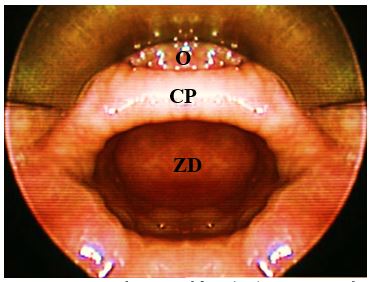
Of surgical importance is that the entire diverticulum is contained within the buc-copharyngeal fascia, which itself is not involved in the formation of the diverticulum (Figure 3). The buccopharyngeal fascia is in effect displaced posteriorly by the posterior wall of the diverticulum, creating a safer plane to divide the “party wall” (that contains the cricopharyngeus) without penetrating the fascia and entering the prevertebral space (Figure 3). This anatomic relationship between the ZD and the surrounding buccopharyngeal fascial layer is the key to understanding how the upper digestive tract remains separated from the retropharyngeal space when incis-ing the anterior wall of the diverticulum, or in the case of an isolated endoscopic cricopharyngeal myotomy. Disrupting this fascial layer could theoretically increase the likelihood of developing mediastinitus.
However when endoscopically dividing a hypertrophic cricopharyngeus muscle in the absence of a ZD, the buccopharyngeal fascia is situated immediately behind the cricopharyngeus muscle, and great care has to be taken to preserve this fascial layer when completely dividing the crico-pharyngeus muscle. Despite initial fears that this fascia could not be preserved during endoscopic cricopharyngeal myotomy, Chang et al demonstrated in a cadaveric study that the buccopharyngeal fascial layer remained histologically intact with carbon dioxide (CO2) laser cricopharyngeal myotomy.4
ZD occurs predominantly in the elderly; it is rarely seen in patients <40yrs of age. A thorough history may raise suspicion of a ZD. Hallmark symptoms are progressive dysphagia coupled with postprandial regurgitation of undigested food. When associated with halitosis, weight loss, noisy deglutition, coughing, globus sensation, and recurrent unprovoked aspiration, the history alone is pathognomonic.5 Symptoms may exist for weeks to years, and the severity of the symptoms generally correlates with the size of the ZD. Physical examination is often normal, although occasionally pooling of saliva in the hypopharynx may be noted on laryngoscopy that may or may not clear with swallowing. A neck mass is rarely palpable. Rarely, Boyce’s sign, or a swelling in the neck that gurgles with palpation, is present.6
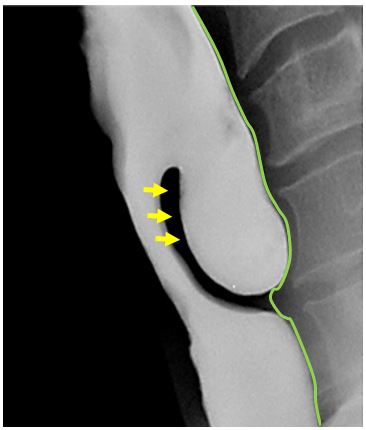
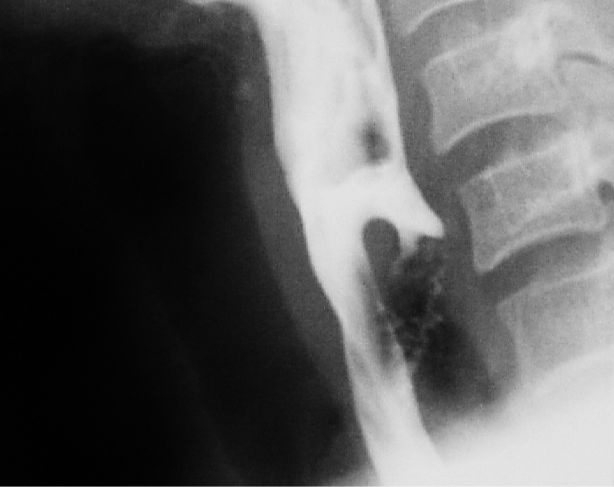
Clinicians must mindful that, although rare (incidence <0.5%), carcinoma can occur in a ZD 7. Blood-tinged regurgitated food should raise suspicion for this, and is an indication that observation is not a reasonable option. Barium swallow frequently shows filling defects within the sac which usually represent undigested food. However, a constant filling defect on serial studies should raise concern of a tumour.5
Diagnosis is confirmed with a contrast swallow and videofluoroscopy (Figure 3). Laryngoscopy is done to rule out other causes of dysphagia, as well as to document vocal cord dysfunction. Preoperative evaluation must include assessment of the function of the lower oesophageal sphincter, as cricopharyngeal myotomy in patients with an incompetent lower oesophageal sphincter places them at risk of developing severe gastroesophageal and laryngopharyngeal reflux.
A ZD is a thin-walled sac lined by stratified squamous epithelium with a thin layer of fibrous submucosa. The muscular layer of the hypopharynx is absent from its wall. The posterior mucosal herniation passes through Killian’s dehiscence/ triangle and is located between the inferior fibres of the inferior pharyngeal constrictor and the superior edge of cricopharyngeus muscle (Figures 1, 3). The common (party) wall contains the cricopharyngeus at its upper edge and separates the oesophagus from diverticulum (Figures 2, 3).
It is important to understand that after the ZD herniates through Killian’s dehiscence, it expands posterior to the cricopharyngeus and the superior oesophageal muscles layers, but is still contained within the peri-oesophageal fascia. This protective layer of investing facia surrounding both the oesophagus and ZD is the key to endoscopic division of the cricopharyngeus muscle and the entire party wall between the ZD and the oesophagus. Without this fascial layer, saliva would be likely to track into the mediastinum causing mediastinitis.
Observation with dietary manipulation is a reasonable option for a relatively small ZD, or in patients with minimal symptoms, or who pose a significant anaesthetic risk.
Surgical approaches include the following:
Most authors currently advocate endoscopic surgery as the preferred treatment, and many studies have reported endoscopic techniques to be both safe and effective.8-12 Advantages compared to external procedures are brief anaesthetic time, early oral intake, short hospitalisation, minimal pain and that revision surgery, either endoscopic or external, is not compromised by scarring.
As with any surgery, the risks and benefits of surgery must be carefully weighed, particularly because ZD patients are generally elderly and often infirm.
Endoscopic procedures
Endoscopic surgery completely divides the common wall between the oesophagus and the diverticulum (diverticulotomy), thereby incorporating the diverticulum into the oesophageal lumen; and in the process also performs a cricopharyngeal myotomy (Figure 4).
Much debate exists regarding the most effective technique. The authors are in agreement with a study that found endo-scopic CO2 laser to be more effective and to result in significantly less recurrence than endoscopic stapling.10 This may be because the endoscopic stapler does not divide the most distal part of the common wall between the oesophagus and the diverticulum (Figure 4). In parts of the world where neither a stapler nor CO2 laser is available, a long suction cautery will work in the place of the laser.
Consideration has to be given to flexibility and disease of the cervical spine. Communication with the anaesthesia care team should include a discussion regarding the need for paralysis as a component of the general anaesthetic, as muscular tone may limit visualisation and complicate placement of the scope.
The choice of operating diverticuloscope depends on the procedure to be performed.
Benjamin diverticuloscope: (Figure 5a) For CO2 laser diverticulotomy, the authors have found the Benjamin diverticuloscope (Storz) to provide the optimum combination of visualisation and ease of placement. Because it has straightened tips, rather than outward-curved tips of the Dohlman scope that was first used for endoscopic surgery, it is often able to be placed in patients in whom placement of the original Dohlman scope would have been difficult.
Weerda diverticuloscope: The long bival-ved Weerda diverticuloscope (Storz) provides additional anterior-to-posterior volume by opening the blades of the scope; this is essential for using the stapler technique. However the width of the proximal end of the scope makes it more difficult to insert in patients with narrow interdental width.
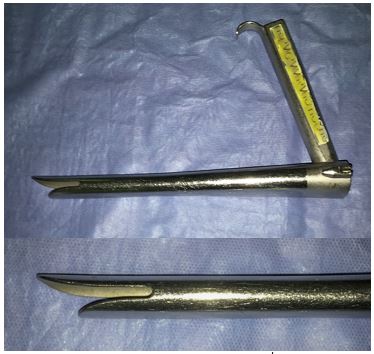

Passing a diverticuloscope can be difficult or impossible in some patients. It is therefore important to caution patients that an endoscopic approach may have to be abandoned due to poor access. In less experienced hands the scope is a particularly dangerous instrument as it may be difficult to insert and may penetrate the posterior pharyngeal wall or the pouch causing cervical sepsis and mediastinitis (Figure 6).
It is therefore crucial that the surgeon exercises sound judgement and abandons the procedure before causing an iatrogenic perforation.
Endoscopic CO2 laser cricopharyngeal myotomy +/- diverticulotomy
CO2 laser has the advantage of dividing the party wall up to the inferior aspect of the pouch. When a stapler is used there is always a residual inferior lip of the pouch that cannot be divided by the device due to its design; the laser is often used to divide this residual bar. In the era of critical cost containment the laser therefore has the advantage of being able to complete the entire task, whereas the stapler may need assistance of further division of the inferior party wall. Asymptomatic postsurgical subcutaneous emphysema occurs slightly more frequently following CO2 laser than with the endoscopic stapler.

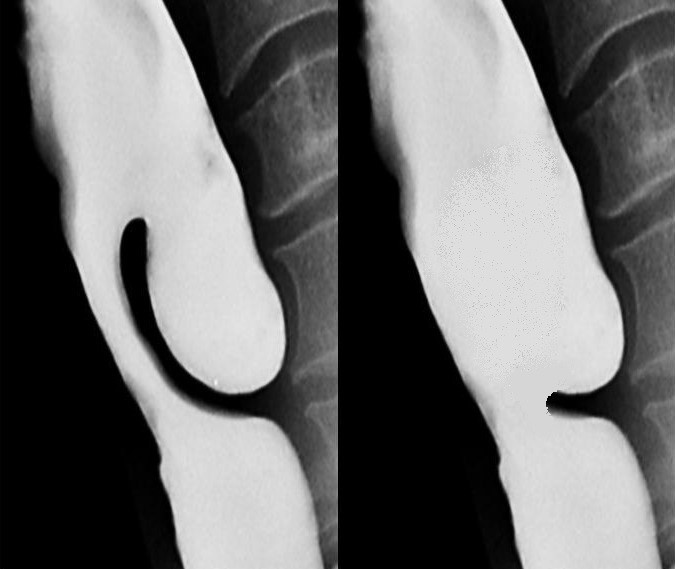
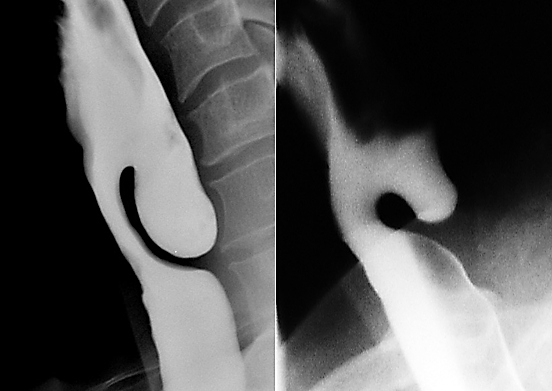
CO2 laser is particularly useful for pouches of <4cms because the stapler does not divide the distal 1-2cms of the party wall (Figure 9).
Cricopharyngeal myotomy forms part of the diverticulotomy procedure as described above, but may also be done in isolation for very small ZDs or for cricopharyngeal spasm
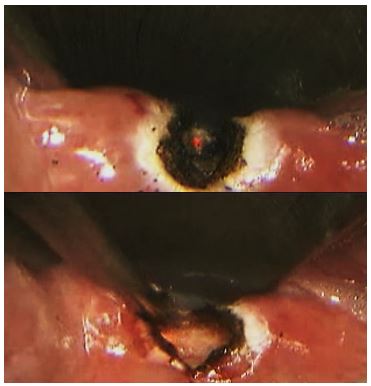
without disrupting the posterior layer of perioesophageal fascia (Figure 10)
Endoscopic stapling, though not our preferred method, is a valuable method to use if CO2 laser is not available.
Compared to laser or diathermy, it is quicker (few minutes), has a lesser risk of perforation and mediastinitis, provides good haemostasis, and avoids thermal injury to the recurrent laryngeal nerve.
The principal disadvantage is that it does not divide the distal 1-2cms of the wall of the diverticulum, and is therefore not suited to small pouches (Figure 9).
The first few procedural steps are identical to the CO2 laser approach described above. The procedures only differ following inspection and stretching of the cricopharyngeus muscle.

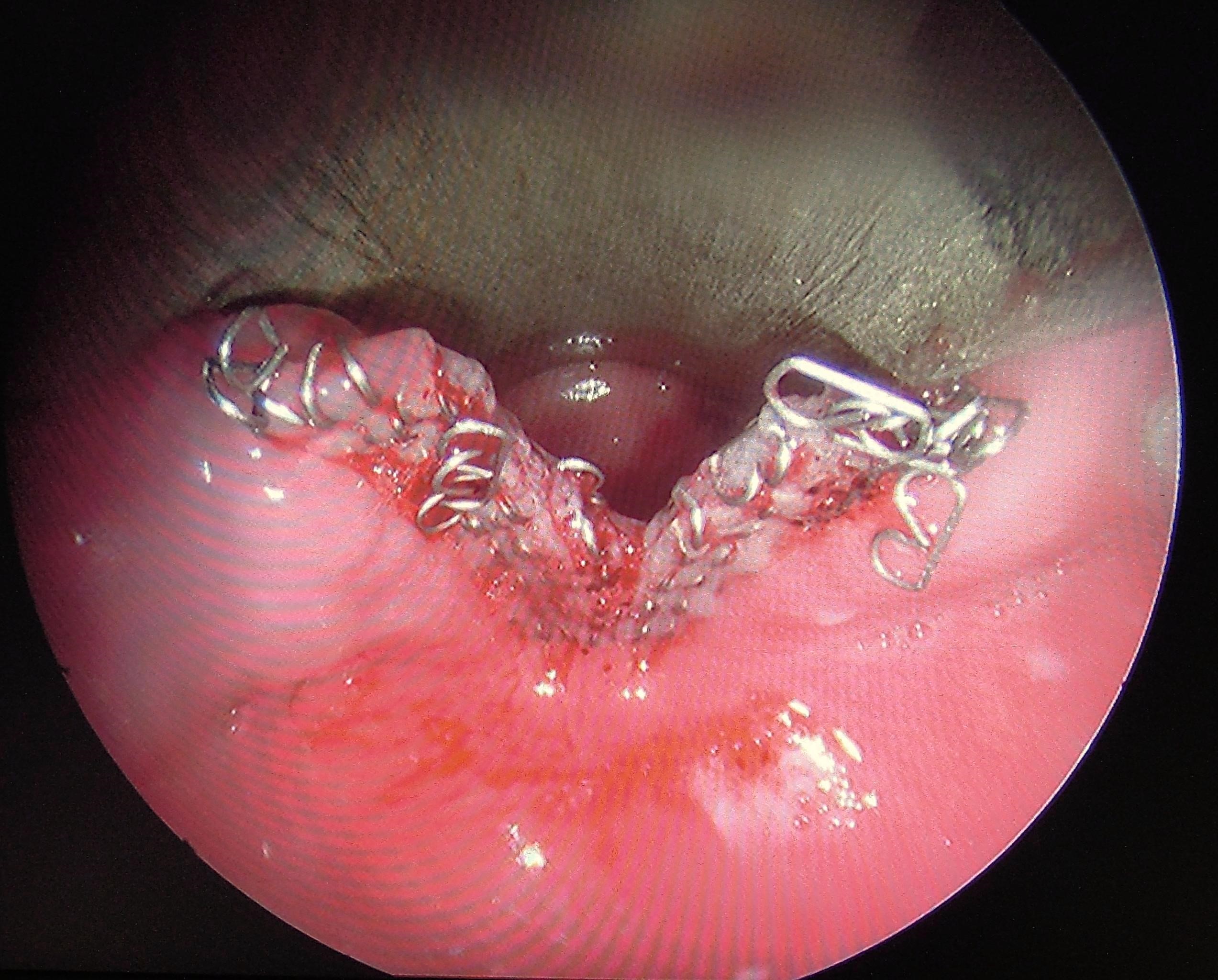
It is important to note that the tip of the stapler cartridge extends beyond the end of the staples, and that the staples extend beyond the razor incision (Figure 13). The result is that this method will always leave some residual sac, which we believe contributes to its higher recurrence rate.

One option to minimise this problem is to place traction sutures on either side of the planned myotomy prior to firing the stapler. Pulling on these sutures during stapler activation maximises the length of the razor cut. Some evidence exists that this may increase the long term success of this technique.13
In resource-limited settings, access to endoscopic staplers and CO2 laser may not be possible due to the significant associated costs. Consequently, we believe that another option for endoscopic treatment of ZD without the expense of this equipment is to use electrocautery to perform diverticulotomy, as was originally described by Dohlman.14 With this technique one exposes the common wall using a diverticuloscope in the same way as with the previous two procedures. A pair of insulated surgical forceps is then used to grasp the common party wall in the midline, and mono-polar electrocautery is applied to the forceps. This is repeated as many times as is necessary to carry the myotomy down to the most inferior aspect of the common wall. Again, sutures may be placed on either side of the myotomy to help close the mucosa, though this is not necessary because the buccopharyngeal fascia, the key layer to maintaining separation between the digestive tract and the mediastinum, remains intact.
The primary indication to treat ZD in an open fashion is inability to gain adequate exposure using transoral endoscopic techniques, or with an extensive, large ZD. Although rare, the anatomy of some patients will prevent adequate endoscopic exposure. As a result, all surgeons who treat ZD endoscopically should be familiar with open transcervical techniques, and should be prepared to perform it if needed. Historically, different open procedures were performed including diverticulopexy and inversion if the pouch. In our experience, success rates are significantly higher with open diverticulectomy.
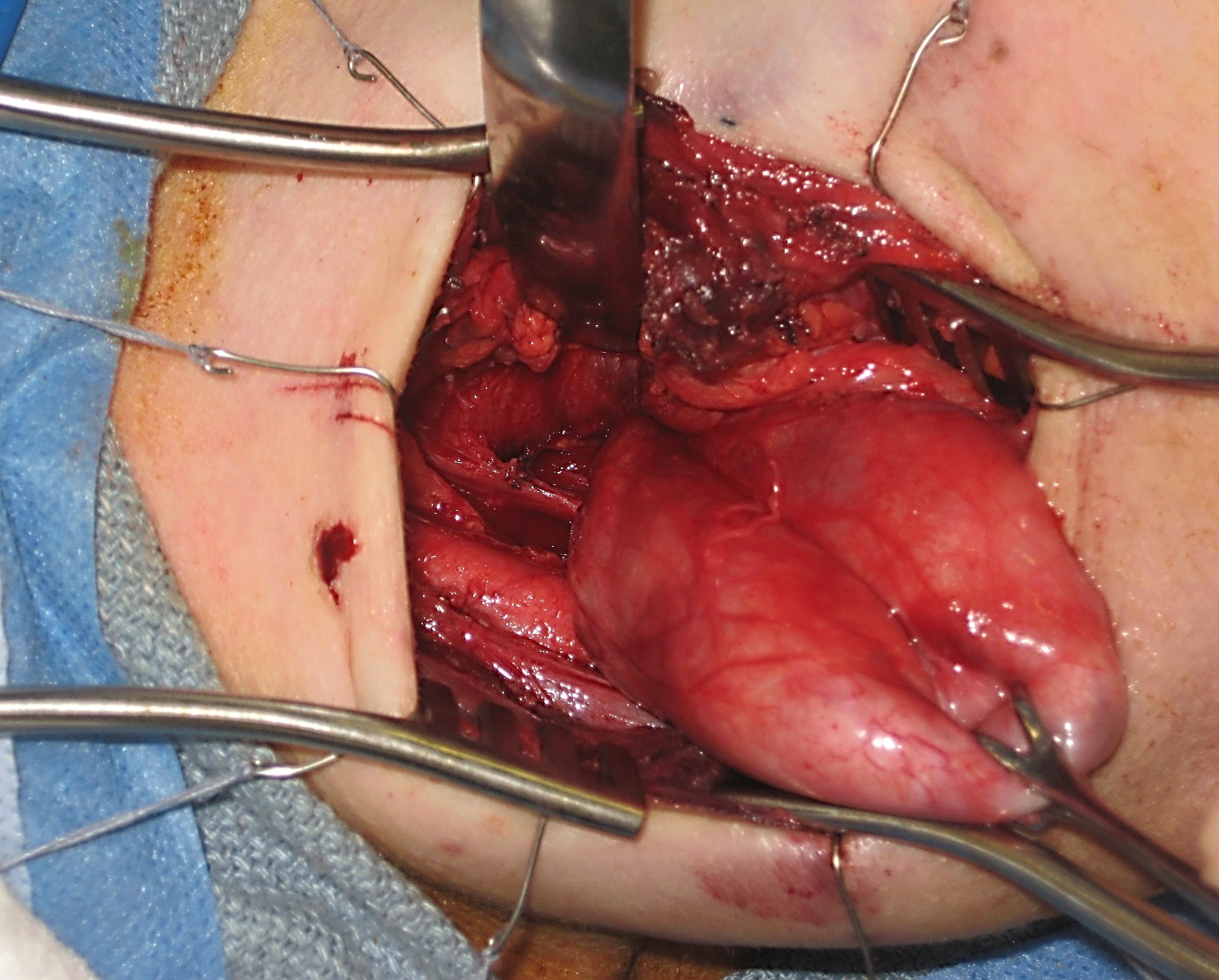
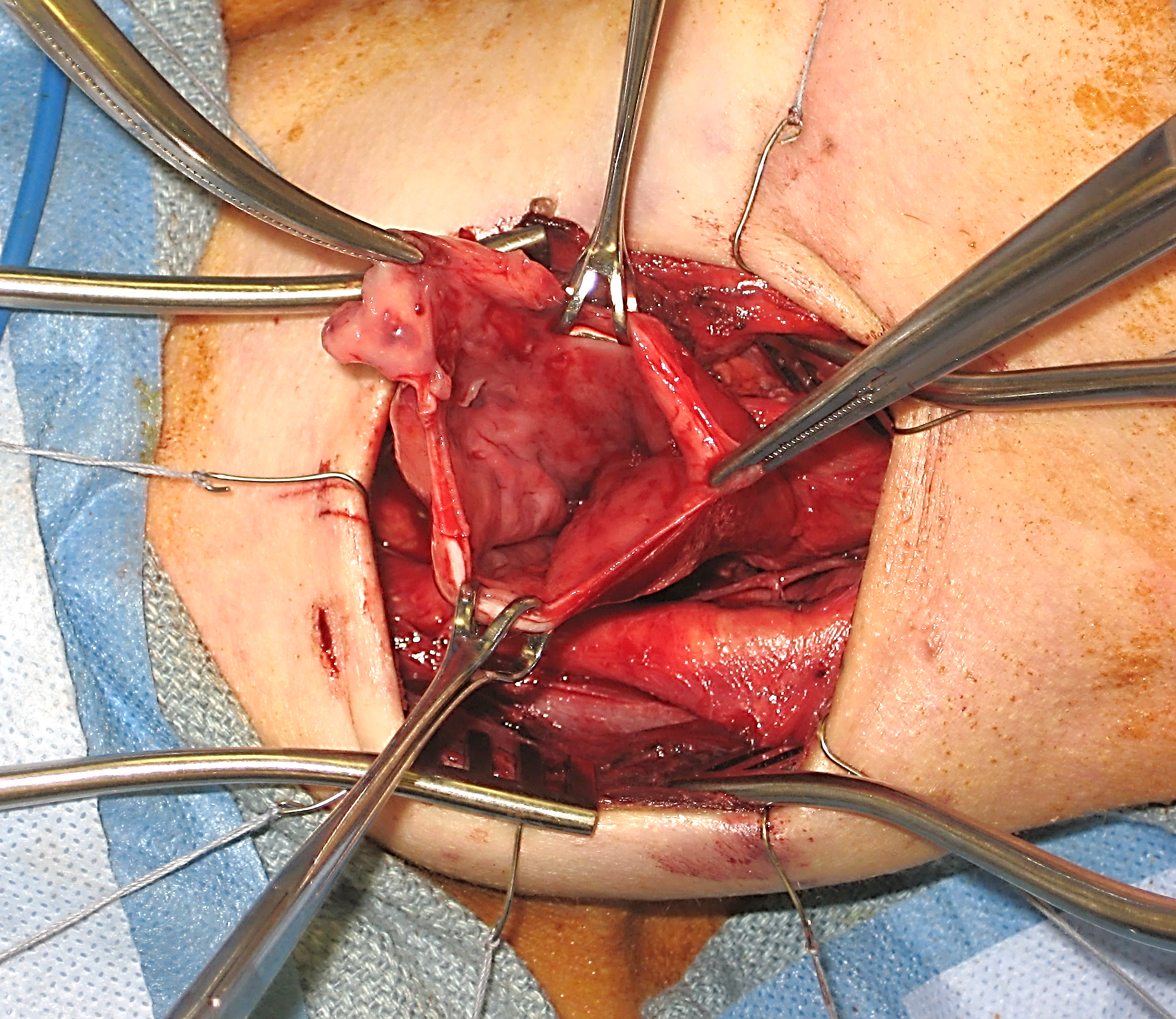
Like all surgical procedures, operations performed for ZD are not without complications. When performing endoscopic procedures, care should be taken to avoid causing damage to the lips, teeth, tongue, and gums especially in cases of difficult exposure. Bleeding is a potentially dangerous complication, as a haematoma in the neck can result in airway compression. As a result, particular attention should be paid to achieving haemostasis during open procedures.
Perhaps the most feared complication of ZD surgery, which can occur in both endoscopic and open procedures, is oesophageal perforation and a subsequent leak. Because the fascial planes in the neck are continuous with the mediastinum, leakage of oesophageal contents into the neck can cause mediastinitis, which can be lethal if not quickly recognised and managed appropriately. In endoscopic procedures, perforation can occur in two main ways. The pouch itself can be perforated by advancing equipment too aggressively. This can also be caused by a stapling device, or an aggressively inserted suction or blunt probe. Alternatively, division of the common wall between the diverticulum and the oesophagus can result in perforation if the dissection is carried too deeply behind the divided muscle layer. In order for a leak to occur, the perioesophageal fascia must be violated. Perforation and mediatinitis should be diagnosed promptly. Early signs include tachycardia, fever, and chest and neck pain. Crepitus may also occur. Any concern about this complication should prompt an immediate workup and empiric therapy. The patient should take nothing by mouth, and broad spectrum antibiotics should be started empirically. Serial chest X-rays may demonstrate expanding subcutaneous air. A contrast swallow should be performed to establish if a clinical fluid leak is present through the perioesophageal fascia. If diagnosed early, this can usually be managed conservatively. A nasogastric tube for feeding may be necessary until the perforation heals.
Open procedures can result in additional complications. Injury to critical structures in the neck are the primary concern. The recurrent laryngeal nerve is of particular interest, and can be identified if needed if the neck of the diverticulum is more inferior than is usual. If the dissection and ligation of the ZD is performed in the midline posteriorly the nerve is naturally avoided during this dissection.
Postoperative wound infection can occur as well, particularly if diverticular contents are spilled into the neck. This underscores the need to suction the sac endoscopically prior to opening the neck.
Excising too much of the sac wall with an open diverticulectomy procedure may cause a stricture.
Regardless of the technique used to treat ZD, perhaps the most common problem is recurrence. In our experience, this is more likely to occur after endoscopic stapling; we feel this is due to the inability to extend the diverticulotomy to the most inferior edge of the sac. In some cases, symptomatic recurrence can progress to the point that a revision procedure is indicated. When recurrence does occur, the diverticulum is often wider. In order to successfully address recurrence, the surgeon must extend the diverticulotomy all the way to the bottom of the sac and eliminate the shelf.
Daniel Schuster, M.D.
Dept. of Otolaryngology - Head and Neck Surgery
Vanderbilt University Medical Center
Nashville, TN
USA
daniel.schuster@vanderbilt.edu
James L. Netterville, MD
Mark C. Smith Professor and Director of Head and Neck Oncologic Surgery
Dept. of Otolaryngology – Head and Neck Surgery
Vanderbilt Bill Wilkerson Center Nashville, TN
USA
james.netterville@vanderbilt.edu
Johan Fagan MBChB, FCORL, MMed
Professor and Chairman
Division of Otolaryngology
University of Cape Town
Cape Town
South Africa
johannes.fagan@uct.ac.za