





Jonathan Oehley, Sudesh Sivarasu
ORCID ID: 0000-0002-7988-7673, 0000-0002-0812-568X
Division of Biomedical Engineering
Department of Human Biology
University of Cape Town
Western Cape
South Africa
Vernon Louw
ORCID ID: 0000-0002-2885-3342
Division of Clinical Haematology
Department of Medicine
Groote Schuur Hospital
University of Cape Town
Western Cape
South Africa
Lynthia Paul
ORCID ID: 0000-0002-5844-0637
Division of Medical Microbiology
Department of Pathology
University of Cape Town
Western Cape
South Africa
Sepsis is a life-threatening condition that occurs when the body’s response to a source of infection is damaging to its own tissues. Sepsis is responsible for substantial health loss worldwide, particularly in sub- Saharan Africa. Increasing rates of antibiotic-resistant bacteria severely reduce the ability of physicians to treat the underlying infection that causes sepsis, especially when the infection is multidrugresistant. Ultraviolet irradiation of the blood was historically used to treat infections.
This project explores this therapy as a novel treatment modality for multidrugresistant sepsis through the development of a device to perform ultraviolet irradiation of the blood. This study reports on the initial findings of the in-vitro irradiation of bacterial-spiked, expired, whole blood with ultraviolet light. Spiked whole blood was exposed to ultraviolet light under different experimental conditions using a custommade testing rig. The initial findings have suggested that the inactivation of bacteria in whole blood may be dependent on a variety of conditions. Further work is being done within this project to identify and quantify these conditions. These findings will then be utilised to guide further testing and development of the device design proposed in this project.
Keywords: sepsis, multidrug-resistant bacteria, whole blood, UVC inactivation, medical device
Sepsis is a life-threatening condition that occurs when a host’s dysregulated response to an infectious organism causes organ dysfunction (Singer et al., 2016). No sepsis-specific treatments currently exist beyond the use of antibiotics (Cohen et al., 2015). Antibiotic therapy is currently the key to reducing morbidity and mortality of sepsis (Thompson, Venkatesh, & Finfer, 2019). However, there is concern that the rate of antibiotic development may not be adequate to address the increasing levels of multidrug-resistant bacteria found in sepsis cases (Goldstein et al., 2019).
Antibiotic resistance has been associated with negative outcomes for patients with sepsis. Sepsis caused by multidrug-resistant bacteria is associated with higher mortality in patients (Capsoni et al., 2019). Additionally, patients with infections that are complicated by multidrug-resistance are more likely to have longer and more costly hospitalisations (Adams, Susi, & Nylund, 2019), highlighting a need for a novel treatment modality for multidrug-resistant sepsis.
Ultraviolet blood irradiation, where blood is exposed to ultraviolet C (UVC) light outside the body, was a therapy used to treat infections over 80 years ago (Hamblin, 2017) . During treatment, blood was withdrawn from the body, exposed extracorporeally to ultraviolet light, and then reinfused back into the patient. Multiple studies in this era reported the resolution or marked improvement of patients with sepsis under ultraviolet blood irradiation treatment (Miley & Christensen, 1947; Rebbeck, 1942, 1951).
The treatment of patients with multidrug-resistant sepsis is a complex problem. However, the effective treatment of spiked blood products could potentially indicate the suitability of a method as a treatment for multidrug-resistant sepsis. Thus, a device for the treatment of infected blood products is under development in this project to evaluate the suitability of UVC irradiation as a therapeutic modality for multidrug-resistant sepsis.
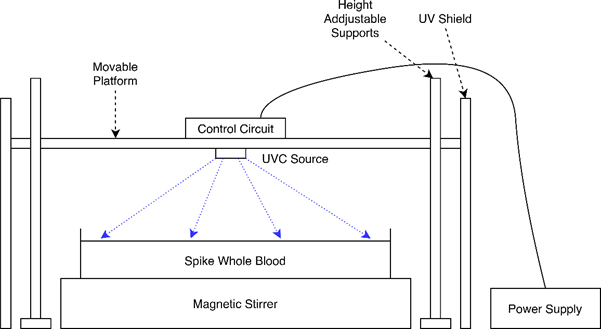
Approvals from both the Human Research Ethics Committee (411/2021) and the Health Sciences Faculty Biosafety Committee (SS/001a/2021-2024) of the University of Cape Town were obtained before initiating the testing in this project.
Expired whole blood was obtained from the Western Cape Blood Services and stored between 2°C and 6°C at the Division of Medical Microbiology Laboratory, at the University of Cape Town. Trypticase soy agar (TSA, Biolab) was prepared according to the manufacturer’s instructions. Staphylococcus aureus ATCC25923 was used to spike the whole blood. The S. aureus isolate was maintained on Brucella agar containing horse blood. For the experiments, the isolate was streaked on blood agar plates and incubated overnight (for 18 to 20 hours). Bacterial cells from the overnight colonies were resuspended in phosphate-buffered saline (PBS) to obtain a concentration of approximately 106 colony forming units per ml (CFU/ml).
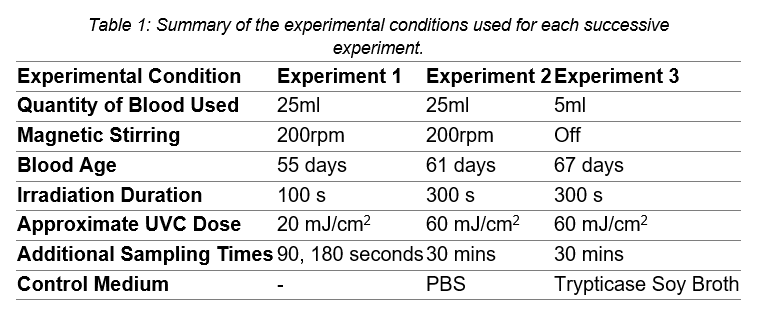
A testing rig (Figure 1) was developed to provide a UVC source for the irradiation experiments. The testing rig's platform was adjusted so that the UVC source was ̴20mm above the surface of the blood. During the experiments, the spiked blood was placed in a Petri dish and stirred on a magnetic stirrer. The experimental conditions for each of the conducted experiments are summarised in Table 1. For each test, samples were taken from the blood before irradiation, after irradiation (plated immediately) and at an additional sampling time after irradiation (Figure 2).
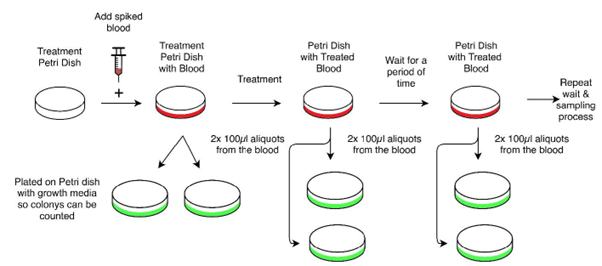
When sampling the blood for measurement, aliquots were serially diluted in PBS and plated in duplicate technical repeats on TSA plates. The TSA plates were incubated at 37°C under aerobic conditions for 24 hours. Colony forming units were then manually counted and recorded. The final colony counts were determined by the average of plates with between 30 and 300 colonies for each sample.
Firstly, these results are from a series of preliminary tests and are intended for internal testing of the methodology and equipment. Thus, any insights and conclusions should be considered preliminary pending further investigation. The blood samples in Experiment 3 were contaminated with other microbes and, thus, there were no quantitative results from Experiment 3. The results from the other two recorded experiments are presented in Figure 3.
Overall, the results in Figure 3 that there was no substantial reduction in the quantity of viable bacteria in the sample either after irradiation or after waiting for an additional delay post- irradiation as specified in Table 1.
Additionally, while the samples in Experiment 3 were contaminated and not counted, it was noted that there was not any discernible qualitative reduction in the quantity of colony growths on the plates. The trypticase soy broth control in Experiment 3 was not contaminated, but also showed no inactivation of bacteria. However, there was complete inactivation of bacteria in phosphate-buffered saline (PBS) control in Experiment 2.
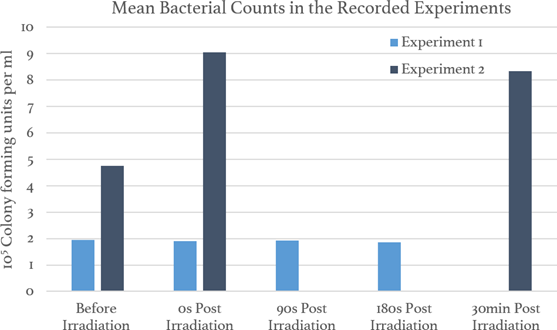
The inactivation of S. aureus has been demonstrated by other studies in various mediums under different conditions (Kim & Kang, 2018; Terpstra et al., 2008). The PBS control in Experiment 2 demonstrated the capability of the experimental setup to inactivate S. aureus under the conditions described in Table 1. Despite this, the bacteria were not inactivated in the whole blood samples or the trypticase soy broth control. This suggests that compounds in these mediums could be absorbing the UVC light and impacting the bactericidal effect of the UVC light.
One of the developers of the historic ultraviolet blood irradiation therapy reported the ability of UVC light to inactivate S. aureus in whole blood (Knott, 1948), and the therapy developed from his work was subsequently used to treat S. aureus infections (Rebbeck, 1942).
Therefore, the lack of inactivation seen so far in this project could be due to the combination of conditions used in these experiments. The experimental conditions which could be affecting the inactivation results are listed in Table 2.
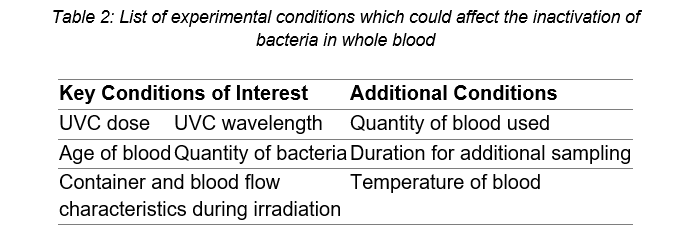
The key conditions of interest all have a large potential to affect the experimental outcome. As it is hypothesised that ultraviolet blood irradiation produces some form of an immune-modulating effect (Boretti, Banik, & Castelletto, 2020), the age of the blood could make a significant difference to the results. This is because various cells of the blood break down over time which could change the immune response of the blood to the UVC radiation. While spiking the blood with high quantities of bacteria (106 CFU/ml) allows for a measurement of the log inactivation achieved by the device, clinically relevant levels of bacteria are much lower at 1-100 CFU/ml (Lamy, Dargère, Arendrup, Parienti, & Tattevin, 2016; Reimer, Wilson, & Weinstein, 1997). Thus, changing the quantity of bacteria used to spike the blood could also affect the results. Finally, the wavelength of UVC light, dose of UVC light, the container holding the blood, and the way the blood flows in the container could all affect how the light is absorbed by the blood and, thereby, the inactivation results. The ongoing work in this project will vary these conditions of interest to attempt to determine the conditions required to inactivate bacteria in whole blood.
The initial findings of this project have shown the ability of the experimental setup to inactivate S. aureus in phosphate-buffered saline in an initial test. However, the lack of bacterial inactivation in the blood samples suggests that the inactivation of bacteria in whole blood may be dependent on a variety of conditions. Further work is being done to identify the relevant conditions and then quantify the effect of these conditions. These findings will then be utilised to guide the further testing and development of the device design proposed in this project.
The authors would like to acknowledge the Western Cape Blood Services for their assistance in the provision of expired whole blood, the members of the Division of Medical Microbiology, UCT who have kindly assisted this project as well as the members of the Medical Devices Laboratory, UCT. The authors would also like to thank Prof. Jonny Peter, Dr Elizabeth Prentice, Dr Sean Wasserman, and the larger working group for the project from UCT and beyond who have assisted and advised during this project.
Adams, D. J., Susi, A., & Nylund, C. M. (2019). Clinical characteristics, risk factors, and outcomes of patients hospitalized in the US military health system with carbapenem-resistant Enterobacteriaceae infection. Am J Infect Control, 48(6), 644- 649. doi:10.1016/j.ajic.2019.10.006
Boretti, A., Banik, B., & Castelletto, S. (2020). Use of Ultraviolet Blood Irradiation Against Viral Infections. Clinical Reviews in Allergy & Immunology. doi:10.1007/s12016-020-08811-8
Capsoni, N., Bellone, P., Aliberti, S., Sotgiu, G., Pavanello, D., Visintin, B., . . . Brambilla, A. M. (2019). Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip Respir Med, 14(1). doi:10.1186/s40248-019-0185-4
Cohen, J., Vincent, J.-L., Adhikari, N. K. J., Machado, F. R., Angus, D. C., Calandra, T., . . . Pelfrene, E. (2015). Sepsis: a roadmap for future research. The Lancet Infectious Diseases, 15(5), 581-614. doi:10.1016/S1473-3099(15)70112-X
Goldstein, E., MacFadden, D. R., Karaca, Z., Steiner, C. A., Viboud, C., & Lipsitch, M. (2019). Antimicrobial resistance prevalence, rates of hospitalization with septicemia and rates of mortality with sepsis in adults in different US states. International journal of antimicrobial agents, 54(1), 23-34. doi:10.1016/j.ijantimicag.2019.03.004
Hamblin, M. R. (2017). Ultraviolet Irradiation of Blood: "The Cure That Time Forgot"? Advances in experimental medicine and biology, 996, 295-309. doi:10.1007/978-3-319-56017-5_25
Kim, D.-K., & Kang, D.-H. (2018). UVC LED Irradiation Effectively Inactivates Aerosolized Viruses, Bacteria, and Fungi in a Chamber-Type Air Disinfection System. Applied and Environmental Microbiology, 84(17), e00944-00918. doi:10.1128/aem.00944-18
Knott, E. (1948). Development of ultraviolet blood irradiation. The American Journal of Surgery, 76(2), 165-171.
Lamy, B., Dargère, S., Arendrup, M. C., Parienti, J.-J., & Tattevin, P. (2016). How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Frontiers in microbiology, 7. doi:10.3389/fmicb.2016.00697
Miley, G., & Christensen, J. A. (1947). Ultraviolet blood irradiation therapy: Further studies in acute infections. The American Journal of Surgery, 73(4), 486-493. Rebbeck, E. (1942). Ultraviolet irradiation of autotransfused blood in the treatment of postabortional sepsis. The American Journal of Surgery, 55(3), 476-486.
Rebbeck, E. (1951). Further studies with ultraviolet blood irradiation therapy (Knott technic) in septic abortions. The American Journal of Surgery, 82(6), 736-740. Reimer, L. G., Wilson, M. L., & Weinstein, M. P. (1997). Update on detection of bacteremia and fungemia. Clinical Microbiology Reviews, 10(3), 444-465. doi:10.1128/cmr.10.3.444-465.1997
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., . . . Angus, D. C. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama, 315(8), 801-810. doi:10.1001/jama.2016.0287
Terpstra, F. G., van 't Wout, A. B., Schuitemaker, H., van Engelenburg, F. A., Dekkers, D. W., Verhaar, R., . . . Verhoeven, A. J. (2008). Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion, 48(2), 304-313. doi:10.1111/j.1537-2995.2007.01524.x
Thompson, K., Venkatesh, B., & Finfer, S. (2019). Sepsis and septic shock: current approaches to management. Internal medicine journal, 49(2), 160-170. doi:10.1111/imj.14199