





Izak Minnie, Stewart Gibson, Neil Stacey
ORCID ID: 0000-0003-0135-4479, 0000-0001-7254-1277, 0000-0003-4902-5201
School of Chemical and Metallurgical Engineering
University of the Witwatersrand
Johannesburg
South Africa
Conventional extracorporeal membrane oxygenation utilises a gas-to-liquid transfer regime to oxygenate blood from a stream of oxygen gas. This work serves as an exploratory study of the liquid-to-liquid transfer of dissolved gases, which may prove advantageous for general applications of ECMO as it allows for higher partial pressures of oxygen as well as the use of isotonic solutions as a means of improving patient haemostability. This concept is of particular interest to an ongoing project which has investigated the use of repurposed hollow fibre membrane dialysers as a means of providing emergency respiratory support using existing equipment. The liquid-liquid regime mass transfer properties of the hollow fibre membrane dialysers were investigated by determining the mass transfer coefficients of carbon dioxide and salt through the membrane as well as the sieving coefficient and leakage rate. Three cartridge types (high flux Braun, low flux Braun and Leoceed 21N) were investigated using a liquid-liquid configuration with carbonated or saline water used as a blood substitute in contact with distilled water. The high flux cartridge demonstrated a high leakage rate making it unsuitable for the proposed use. The two low flux cartridges demonstrated adequate carbon dioxide removal of 75.8% and 64.4% of the recommended 200ml/min for the Braun and Leoceed cartridges, respectively. All three cartridges displayed sieving coefficients close to one, indicating a high rate of salt transfer and subsequent need for solute balance in the final application. The results of this work showed a higher mass transfer coefficient for the liquid-liquid transfer regime compared to prior studies using a gas-liquid transfer regime. This result suggests that a liquid-liquid configuration is not just useful for emergency respiratory support but could outperform existing ECMO techniques. Further study into the use of liquid-liquid HFMD for respiratory support, using blood and conditions more representative of the final intended use, is warranted.
Keywords: Hollow Fibre Membrane Dialysers; Carbon Dioxide Mass Transfer Coefficient; Liquid-Liquid Mass Transfer; Gas-Liquid Mass Transfer; Salt Mass Transfer Coefficient; Leakage.
The ongoing COVID-19 pandemic has resulted in considerable challenges regarding planning and resource management in the healthcare sector (Mirco et al., 2020). Specifically, the provision and application of respiratory support on a global scale has proven difficult (Lyons & Callaghan, 2020).
Respiration is the addition of oxygen to the circulatory system and concurrent removal of carbon dioxide. This gas exchange is important for most bodily functions and if it is not fully functional can lead to a variety of problems regarding hypoxemia or hypoxemia (insufficient or excessive oxygen levels) and hypocapnia or hypercapnia (insufficient or excessive carbon dioxide levels) (Fasano & Sequeira, 2017). Hypoxemia is of particular concern as it can result in cellular hypoxia, organ dysfunction, and death (Martin & Grocott, 2013) through the excessive production of inflammatory cytokines that further worsen lung injuries (Hojyo et al., 2020; Ragab et al., 2020). Hypercapnia can also worsen existing lung injuries through immunosuppression (Takahashi et al., 2018).
One respiratory problem of concern is acute respiratory distress syndrome (ARDS). ARDS is caused by fluid build-up in the alveoli, decreasing the amount of oxygen uptake and leading to hypoxemia (Mayo Clinic, 2020). Pre-COVID-19, ARDS was responsible for roughly 10% of ICU admissions in the United States of America and had a high mortality rate (Claar & Hyzy, 2017). With the onset of the pandemic, between 15% and 30% of COVID-19 patients developed ARDS leading to a substantial increase in hospital admissions and subsequent equipment shortages (Maclaren et al., 2020) as severe COVID-19 cases required ventilatory support (Dondorp et al., 2020).
Respiratory support can be provided using several methods such as high flow nasal oxygen (HFNO), endotracheal intubation, continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), non-invasive ventilation (NIV), and equipment such as face masks, nasal cannula, hyper oxygenated oxygen chambers and mechanical ventilators (Gorman et al., 2021; Matthay et al., 2020; Pfeifer et al., 2020; H. Xu et al., 2011).
While the above-mentioned methods can provide respiratory support in many cases, there are certain situations or potential problems that could preclude their use. These include the uncertainty regarding increased risk of infection to healthcare workers when administering some treatments such as HFNO (Lyons & Callaghan, 2020; Pfeifer et al., 2020), lung injury caused by ventilators (Maclaren et al., 2020) such as barotrauma, atelectrauma, and oxytrauma (Dondorp et al., 2020), the toxicity of oxygen when breathed at high concentrations for an extended period of time (Martin & Grocott, 2013), high morbidity and mortality of invasive ventilation (Munshi & Hall, 2021), high failure rates of non-invasive ventilation for viral influenzas and coronaviruses (Gorman et al., 2021), inefficiency in treating some respiratory diseases such as pneumoconiosis and pulmonary fibrosis (H. Xu et al., 2011) and mechanical ventilation causing hypercapnia (Takahashi et al., 2018).
In the case where a patient is not responding to conventional treatments, extracorporeal membrane oxygenation (ECMO) can be used to provide respiratory support (Pfeifer et al., 2020; Ramanathan et al., 2020). During the COVID-19 pandemic, ECMO has been recommended for severe cases as it has been shown to reduce mortality and improve quality of life (Maclaren et al., 2020; Matthay et al., 2020; Mishra et al., 2010; Ramanathan et al., 2020).
ECMO acts as a cardiopulmonary bypass, where deoxygenated blood is removed, pumped through an artificial membrane lung where gas exchange occurs, and then returned (Maclaren et al., 2020). The most common ECMO membrane is a hollow fibre format polymethyl pentane (PMP) membrane that separates the blood from the gas stream, providing oxygen and removing carbon dioxide (Evseev et al., 2019; Yeager & Roy, 2017). While the primary function of the ECMO method is the oxygenation of blood, requiring a blood flow of 3-6L/min for acceptable oxygenation, in a low flow set-up, carbon dioxide removal can still be achieved (Gattinoni et al., 2019; Makdisi & Wang, 2015; Sidebotham et al., 2012). ECMO can be set up in two ways: veno-venous (VV) or veno-arterial (VA) differing in where the oxygenated blood is returned to the patient (Fasano & Sequeira, 2017). VV ECMO only provides support to lung function and is the more common application, while VA ECMO provides both lung and heart capabilities (Sidebotham et al., 2012). The gas transfer in ECMO is significantly affected by the blood flow through the equipment, additionally, oxygen transfer is determined by pre-membrane oxygen saturation and pre-membrane carbon dioxide levels while the carbon dioxide transfer is determined by the gas flow, haemoglobin levels, and pre-membrane carbon dioxide levels (Park et al., 2013).
While ECMO is proven to be an effective form of respiratory support, it is resource-intensive, highly specialised, and expensive (Maclaren et al., 2020; Mishra et al., 2010). This, coupled with the planning and personnel training required, means that ECMO facilities are restricted to advanced care centres (Matthay et al., 2020; Ramanathan et al., 2020). The Extracorporeal Life Support Organization (ELSO), a global organisation focused on education, training, research, and management of extracorporeal life support, lists only South Africa and Egypt as registered ECMO centres on the African continent (ELSO, 2021). Due to its high resource use and limited availability, ECMO is only recommended for use in essential cases and when all traditional methods have been attempted ( Shekar, 2022; Maclaren et al., 2020).
In resource-poor environments where ECMO capabilities are not available, it has been proposed to use hollow fibre membrane dialysers for respiratory support as dialysis is a widely used technique, with existing clinical approval and a lower resource cost (Rubin, Stacey, Matambo, Vale, et al., 2020). Dialysers are used for haemodialysis therapy and continuous renal replacement therapy (CRRT) where they replace kidney function by filtering small and medium toxins and managing electrolyte and acid balances in the body (Tange & Yoshitake, 2019; ter Beek et al., 2020).
The workings of a dialyser and ECMO machine are similar as both use a hollow fibre design (Yeager & Roy, 2017) with a membrane to separate blood from the secondary exchange flow. The differences are in the membrane type, flow rate, and counter-current flow. HFMD commonly uses a polysulfone membrane that is more permeable than membranes used in ECMO as it is required to exchange not just gas but larger toxins and fluids as well (Tange & Yoshitake, 2019; ter Beek et al., 2020). Dialyser cartridges are also smaller than ECMO resulting in a lower blood flow which means a single cartridge set-up is not capable of providing full oxygenation of blood (Rubin, Stacey, Matambo, Vale, et al., 2020; Sidebotham et al., 2012). This smaller size and subsequently lower blood prime volume could be an advantage of HFMD cartridges as it avoids issues such as requiring blood transfusions that accompany the large priming volume of ECMO (Gimbel et al., 2016). Lastly, HFMD works with a liquid dialysate and not a gas stream as used in ECMO (Rubin, Stacey, Matambo, Vale, et al., 2020).
During the application of conventional dialysis, it has been found that the partial pressure of oxygen in the blood passing through the dialyser had increased (Tange & Yoshitake, 2019). Simulated dialysis using bovine blood has also shown that oxygenating the dialysate results in increased levels of blood oxygenation (Tange et al., 2012; Tange & Yoshitake, 2019). However, there is contradictory evidence on the effect of dialysate oxygenation on blood carbon dioxide levels (Tange et al., 2012; Tange & Yoshitake, 2019). A combination of membrane lung, hemodiafiltration, and acid addition has been shown to remove carbon dioxide from blood (Takahashi et al., 2018).
In terms of respiratory support, CRRT has been shown to improve oxygenation in paediatric patients with ARDS (Yang et al., 2016), and using a gas stream as in ECMO, oxygenation of blood has been achieved using HFMD (Rubin, Stacey, Matambo, Vale, et al., 2020). The latter has been proposed as a means of providing partial respiratory support to COVID-19 patients suffering from a respiratory deficit. It was found that a single HFMD cartridge, with 500ml/min flowing through it, would be capable of supplying as much as 15% of a patient's oxygenation requirements, thereby potentially compensating for even a severe respiratory deficit with a setup that more closely resembles dialysis, an outpatient procedure, than a highly intensive medical intervention such as ECMO.
Oxygenation by a gas stream in HFMD cartridges appears to hold promise as an emergency measure and as a means of retaining reserve healthcare capacity for respiratory support, but also poses certain issues, including possible leakage due to the permeable nature of its membrane (L. Xu, 2020). This, coupled with the low blood flow rates and oxygen partial pressure limitations in pressurised gas and saturated water/dialysate, means HFMD is generally insufficient for full respiratory support. Furthermore, the gas configuration can lead to emboli. Although not particularly dangerous in the VV configuration, as emboli can be filtered when passed through the lung, emboli can cause serious harm in a VA configuration. It must be noted that emboli comprised of pure oxygen are likely to be far more easily absorbed into the bloodstream than air emboli comprised mainly of nitrogen, and therefore the risk of emboli in the VV configuration is reduced by the intrinsic properties of the method and, with proper handling and monitoring, is unlikely to be a severe threat. Nevertheless, it cannot be discounted entirely, nor can the possibility of membrane fouling be occurring as a result of foaming at the interface.
The proposed novel approach to ECMO involves a highly oxygenated liquid stream to be brought into contact with the bloodstream to supply oxygen and remove carbon dioxide. Operationally, this approach could prevent the serum leakage associated with ECMO through one of two means. Firstly, if a hydrophobic oxygencarrying liquid is used, then water and water-soluble blood components will not readily be transferred into the oxygen carrier stream. Secondly, if a hydrophilic or water-based oxygen carrier is used, then it can be formulated to be isotonic in nature, thereby eliminating the osmotic driving force for leakage and ion loss.
Another potential advantage of using a liquid oxygen carrier is that it could be oxygenated at pressures significantly exceeding atmospheric pressure, resulting in a supersaturated solution with an oxygen partial pressure greater than can be achieved in gas-liquid configurations, which are fundamentally limited by the need to keep transmembrane pressure low to avoid mechanical stress on the membrane material.
The most promising candidates for liquid oxygen carriers are Perfluorocarbons and an aqueous solution of Hemopure®. Perfluorocarbons (PFCs) are a category of hydrophobic molecules comprised of Carbon and Fluorine atoms, with an ability to store and release copious amounts of oxygen (Carroll, 2021; Jägers et al., 2020; Tawfic & Kausalya, 2011). PFCs have an oxygen capacity 40 times larger than water and twice that of blood (201 mlO2/LBlood (Pittman, 2011)) and an even larger carbon dioxide capacity (Jägers et al., 2020).
Hemopure® is an aqueous solution containing bovine-derived hemoglobin, able to absorb oxygen in the same manner as blood does. Each of these candidates have advantages and disadvantages. For Hemopure®, it is not possible to usefully elevate the partial pressure of oxygen above atmospheric pressure. This is because Hemopure® follows a similar oxygen-dissociation curve to that which characterises blood, and for which the capacity to absorb oxygen plateaus at higher partial pressures as the active sites on the haemoglobin become saturated. Perfluorocarbons, by contrast, exhibit a Raoult's Law behaviour where there is a strong linear relationship between oxygen partial pressure and dissolved oxygen content, thereby allowing for super-saturation beyond partial pressures of one atm. Hemopure®, on the other hand, is advantageous because it is an aqueous solution and thus lends itself to the addition of other solutes for the benefit of patient stability.
However, the current study is a preliminary investigation of membrane gas transfer in the liquid-liquid configuration and therefore selecting an optimal oxygen carrier is outside the scope of work undertaken at this stage. Further, the operational and ethical requirements of experimentation with blood are more onerous than appropriate for an initial pilot study. The principal area of concern in this study is whether gas transfer takes place in the liquid-liquid configuration, or whether the gas transfer is obstructed by liquid filling of pores. Hence, the primary goal is to estimate a mass transfer coefficient for a convenient respiratory gas, to determine whether it is comparable to the mass transfer coefficient for the same gas in the gas-liquid configuration. For reasons of convenience, carbon dioxide is chosen as the studied gas, and water is used as the solvent. This results in an inexpensive experimental setup with minimal operational obstacles, which is therefore easily reproducible for corroboration or new testing with alternative membrane materials.
The basic experimental set-up can be seen below in Figure 1.
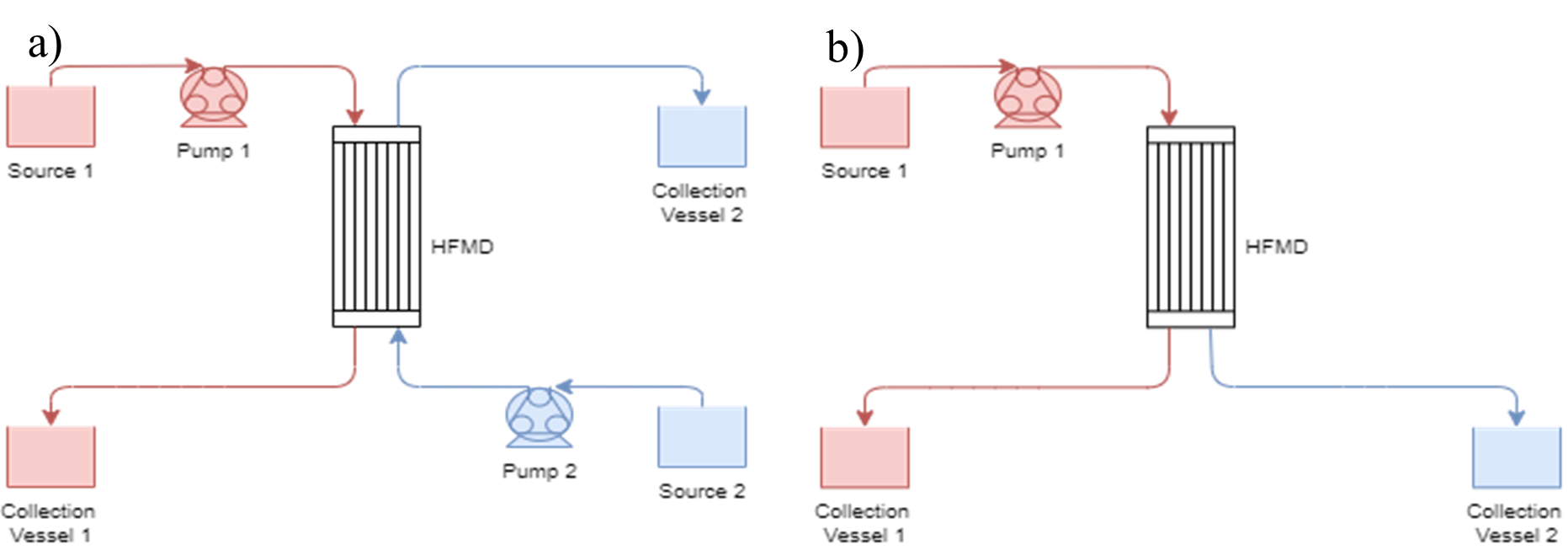
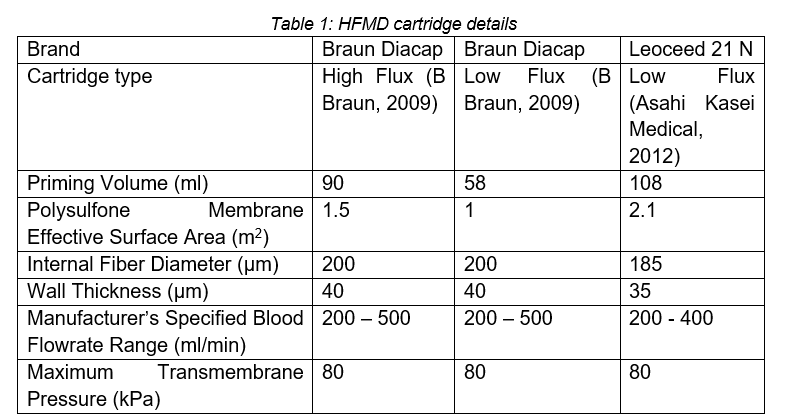
As seen in Figure 1a, the set-up uses two pumps: the tube-side pump in the red circuit runs at around 500ml/min and the shell-side pump in the blue circuit runs at around 1000ml/min. Unfortunately, due to equipment constraints, flow control of the pumps was limited, especially with the fish pump used on the shell side. Three HFMD cartridges were considered, the details of which can be seen in Table 1.
The liquid and conditions used in each loop were varied based on the experimental parameters required.
Carbon dioxide is an important gas that needs monitoring during oxygen therapy. The normal partial pressure of carbon dioxide in arterial blood is between 35- and 45- millimetres of mercury (Messina & Patrick, 2021). As mentioned, high (greater than 45mmHg) and low (less than 35mmHg) levels of carbon dioxide can have severe effects on the body (hypercapnia and hypocapnia). Therefore, the mass transfer of carbon dioxide in a HFMD is important as it will determine whether the use of HFMD for oxygen therapy is viable or not.
To evaluate the mass transfer coefficients of carbon dioxide across the HFMD membrane, carbonated distilled water (pH 3.9 – 4.2) was run through the red loop while distilled water was run through the blue loop (Figure 1a). After a priming period, the carbon dioxide content at each inlet and outlet of the HFMD cartridge was determined using a pH meter along with the Acid-Base equations below.
Seventy per cent of carbon dioxide is transported through an acid-base, catalysed interconversion between carbonic acid (H2CO3), bicarbonate (HCO3-), and hydrogen (H+) ions [14]. The acid-base equation can be given below with the equilibrium constant, K, given by (Boyd, 2015):
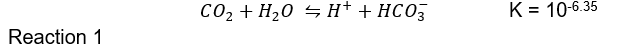
The equilibrium expression of the reaction above can thus be written as follows:
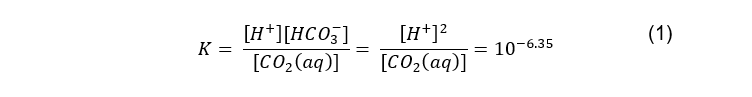
and as pH is defined as:
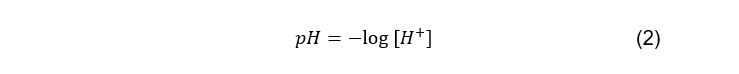
The pH of water can be used to determine the dissolved carbon dioxide concentration.
The mass transfer coefficient of both gases through the membrane can be modelled using the following equation:
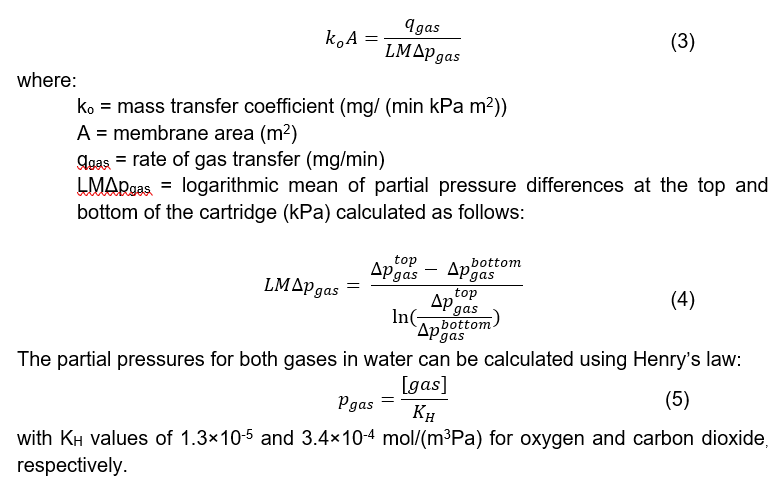
Since the primary use of HFMDs is to remove toxins from the blood (Khandpur, 2020), special attention needs to be paid to the transfer of electrolytes when using the membrane for respiratory support. A significant drop in a patient’s electrolyte level, known as hyponatremia, may cause headaches, dizziness and, in severe cases, lack of consciousness. It is imperative to consider this as it will justify the need for an electrolyte support solution if there is excessive ion loss from the blood to the dialysate. To evaluate the mass transfer coefficients of NaCl across the HFMD membrane, salt was dissolved in distilled water (6 – 12g/L) and run through the red loop while distilled water was run through the blue loop (Figure 1a). At each inlet and outlet of the HFMD cartridge, the salt content was determined using an EC/TDS meter.
The salt mass transfer coefficient can be determined through a minor adjustment to Equation 3 by determining the log mean concentration instead of the log mean pressure.
The sieving coefficient is the measure of the equilibration of a substance through a semipermeable membrane (Kashani et al., 2012). Simply put, the sieving coefficient is the measure of how easily a substance passes from the blood plasma to the filtrate in a HFMD. The sieve coefficient equation is given by the following equation (Neri et al., 2016):

A sieving coefficient of one indicates that the substance equilibrates on both sides of the membrane and a sieving coefficient of zero indicates that the substance does not cross the membrane at all.
To determine the sieving coefficient, a salt solution as prepared by dissolving table salt (NaCl) in distilled water (10g/L). This water was run through the hollow fibers of the cartridge in a closed-loop configuration with no opposing gas or liquid flow (Figure 1b). After allowing time for priming of the system, the leakage was collected from the shell-side of the cartridge. Using an EC/TDS meter, the total dissolved solids and electrical conductivity of the reservoir and collected leakage were determined.
Hollow fibre membrane dialysers are specifically designed for the transfer of toxins from the blood to the dialysate through the mixing of fluids (Khandpur, 2020). However, during this mixing, some of the blood remains in the dialysate and vice versa, this is known as leakage. Leakage is synonymous with bleeding as blood is lost from the body. If too much blood is lost, the body goes into hypovolemic shock, which impedes the heart's ability to pump sufficient blood (Machalinski, n.d.). As a result, tissues do not receive enough oxygen, leading to tissue damage.
To evaluate the leakage of fluid from one side of the membrane to the other, the two loops were run in three configurations for each cartridge:
The results are presented with outliers removed based on the IQR method. Most of the outliers removed had flowrates significantly different to the average of the run and this is likely the cause of their outlying values. Results are reported to be ± 1 standard deviation.
The rate of carbon dioxide removal in HFMD depends on membrane area, pore size, and pore material. It was found that membrane fibres with small pores and hydrophobic construction are better suited for carbon dioxide absorption (Sumin et al., 2010). All three cartridges, Braun Low Flux, Braun High Flux, and Leoceed21N, comprise of a polysulfone membrane, which has a hydrophobic nature (Espinoza- Gómez & Lin, 2003). Therefore, it is expected that the two low flux cartridges have higher carbon dioxide mass transfer coefficients than the high flux cartridge. Additionally, one would expect the mass transfer for a species across the membrane to be constant as the membrane area, pore size and pore material should be constant. The carbon dioxide mass transfer coefficients as a function of initial carbonated source pH are shown in Figure 2.
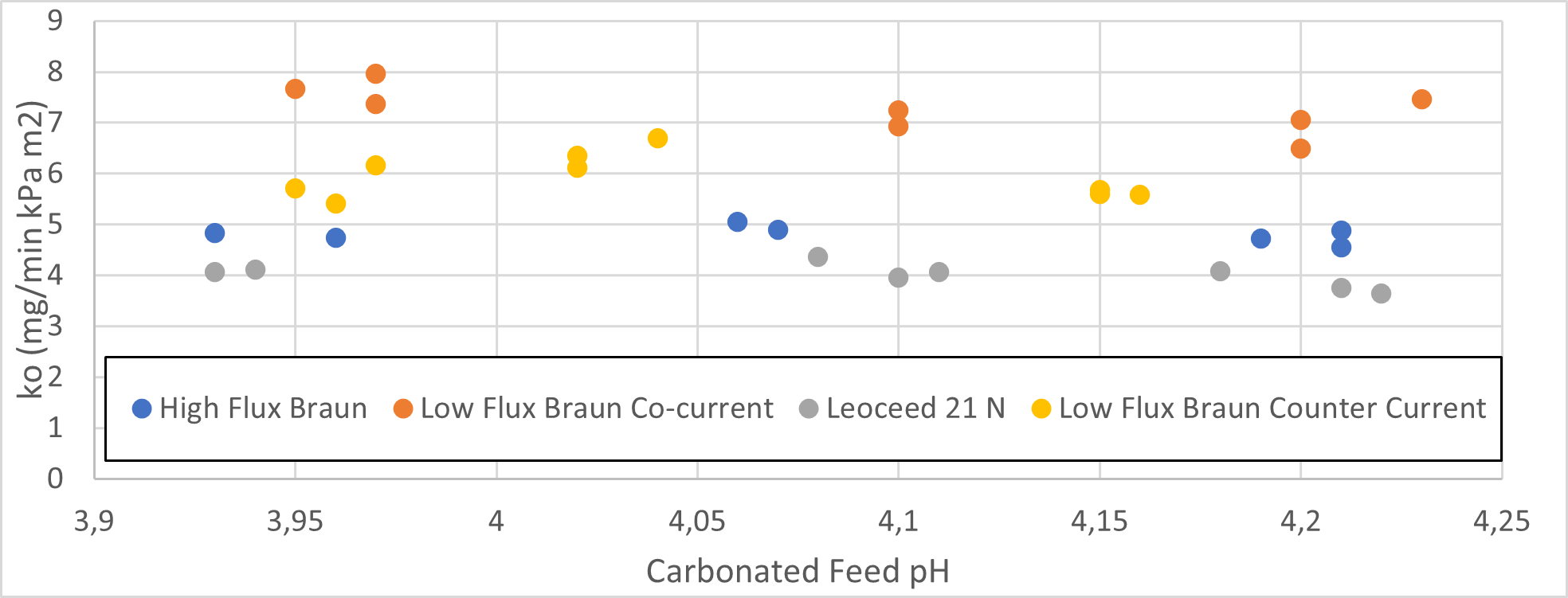
The low flux Braun cartridge in the co-current configuration resulted in the highest mass transfer coefficient, while the Leoceed 21N resulted in the lowest. A summary of the average mass transfer coefficients for each cartridge can be seen in Table 2.
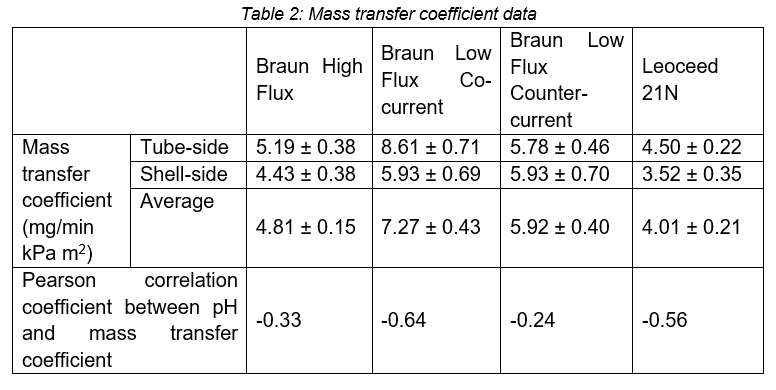
The difference in the mass transfer coefficient seen between the shell- and tube-side data is due to a mass balance inequality where the mass of carbon dioxide lost from the tube-side is higher than the mass gained by the shell-side. This discrepancy is most likely due to two systematic errors. Firstly, the measurements made were discrete and not continuous which made it difficult to see whether equilibrium had been reached when the measurement was taken. This implies that equilibrium had not yet been reached and that some of the missing carbon dioxide had been adsorbed onto the membrane. Secondly, the time between collection and pH measurements of the liquid discharge on both sides, coupled with insufficient equipment to fully cover the collection vessels, resulted in the off gassing of carbon dioxide on both sides. This off-gassing would increase the pH measurement indicating that the tube-side lost more carbon dioxide than in reality and, simultaneously, that the shell-side gained less carbon dioxide. Due to these reasons, the average mass transfer coefficient is considered the most reliable.
The Pearson correlation coefficient shows a slight negative correlation between pH and mass transfer coefficient across all cartridges, with the effect being most pronounced in low flux co-current set-ups. This is a surprising result as we expect the coefficient to remain constant regardless of initial concentration. However, this result must be investigated further as it is derived from a limited set of data points (n = 7 – 9) for each cartridge and could be a result of flowrate variations that can influence the mass transfer coefficient as found by Lan Xu (L. Xu, 2020).
While the carbon dioxide mass transfer coefficient data is a valuable piece of information regarding the use of HFMD in gas transfer applications for use in a clinical setting, the much more relevant information is the rate of carbon dioxide removal as that will dictate whether the device can prevent hypercapnia in patients requiring respiratory support.
Using ECMO as a basis for comparison, membrane oxygenators aim to remove 200ml CO2/min for an average adult (Baker & Low, 2015). The carbon dioxide removal rate found in this project can be seen in Figure 3. The graph illustrates that all four configurations display exponential relationships between carbon dioxide removal and pH. The equations and R2 values of each cartridge can be seen in Table 3.
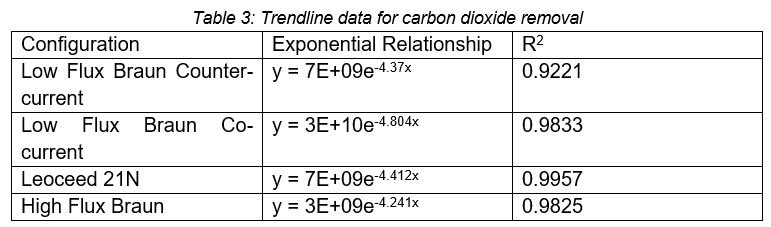
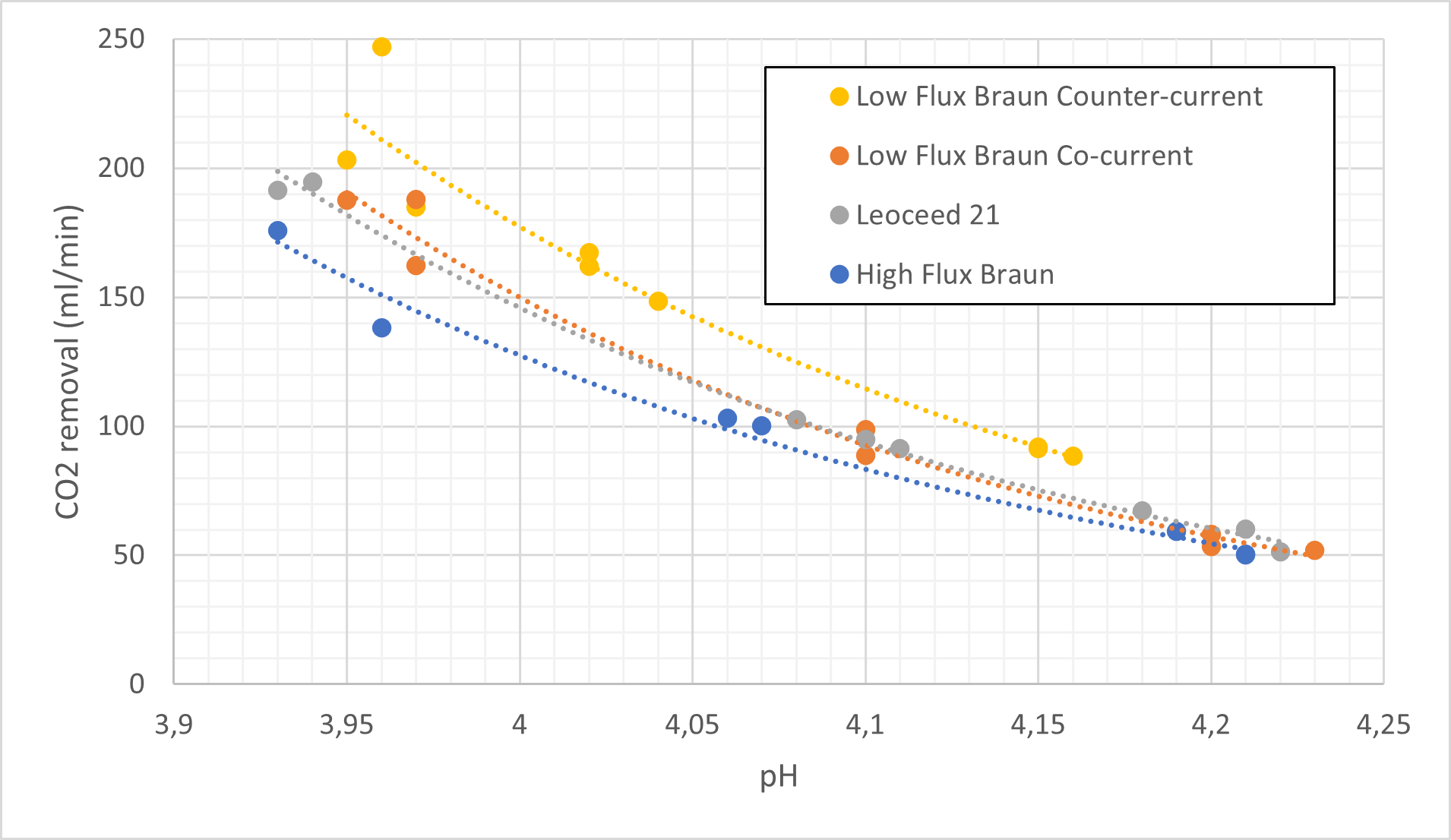
At low pH values (3.95) the HFMD cartridges come close to fulfilling the carbon dioxide removal requirements with >75% removal in all cases. However, it decreases to only around 33% at higher pH values (4.2).
The carbon dioxide partial pressure in venous blood is 6.1kPa (Arthurs & Sudhakar, 2005), using equations 1, (2, and (5 this corresponds to a pH of 4.52. This is a much higher pH than the range considered during experimentation, and due to the differences between blood and water, a direct comparison is not entirely valid, but it can serve as a broad indication of the expected performance of the HFMD cartridge in a clinical setting. Using the relationships presented in Table 4, the percentage of required carbon dioxide removal achieved by each cartridge can be seen below.
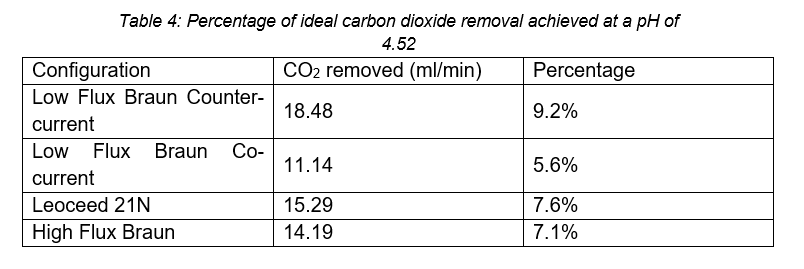
Based on the carbon dioxide removal results presented, multiple HFMD cartridges would be required to achieve even partial respiratory support.
The work presented is a variation of work done by Lan Xu in 2020, where the carbon dioxide mass transfer coefficient and carbon dioxide removal of HFMD cartridges was investigated using a liquid-gas configuration (L. Xu, 2020). The work done by Xu focussed on the Low Flux Braun and Leoceed 21N cartridges run at a pH of 4 with varying flow rates. A comparison between the results of that study and the present work is presented in Table 5, calculated from trendline data based on a pH of 4 and flowrate of 500ml/min.
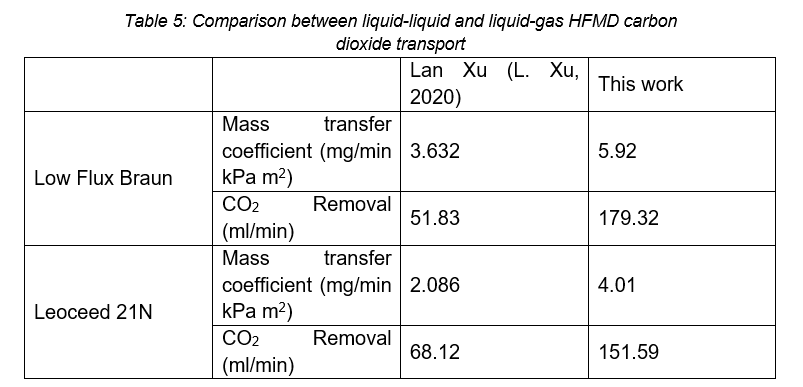
The difference in the Leoceed21N results may be due to the configuration. This paper investigated co-current flow, whereas Xu (L. Xu, 2020) investigated countercurrent flows, indicating that co-current flow has a higher leakage rate than countercurrent flow. However, the difference is also due to the difference in exchange medium. This can be further confirmed by comparing the low flux Braun countercurrent mass transfer coefficients, indicating that liquid-liquid exchange has a higher mass transfer than gas-liquid exchange.
This increase in carbon dioxide transfer as well as the presence of a suitable medium to prevent or balance solute losses as explained in subsequent sections means the liquid-liquid configuration is preferred for the application of emergency HFMD respiratory support. The improvements in performance shown by a liquid-liquid configuration also have significant implications for the ECMO field as current ECMO techniques implement a gas-liquid configuration. Thus, patients using ECMO can experience better respiratory support, by simply replacing the gas stream with an oxygen-carrying support fluid. Fluids with high oxygen-carrying abilities, such as perfluorocarbons and Hemopure® solutions, can be used.
The results for the salt mass transfer coefficient were handled in an equivalent way to the carbon dioxide results, with the exception that the results presented were based solely on the tube-side mass transfer and not the average. This was done as membrane adsorption and lack of time to equilibrate caused large deviations in the shell-side results.
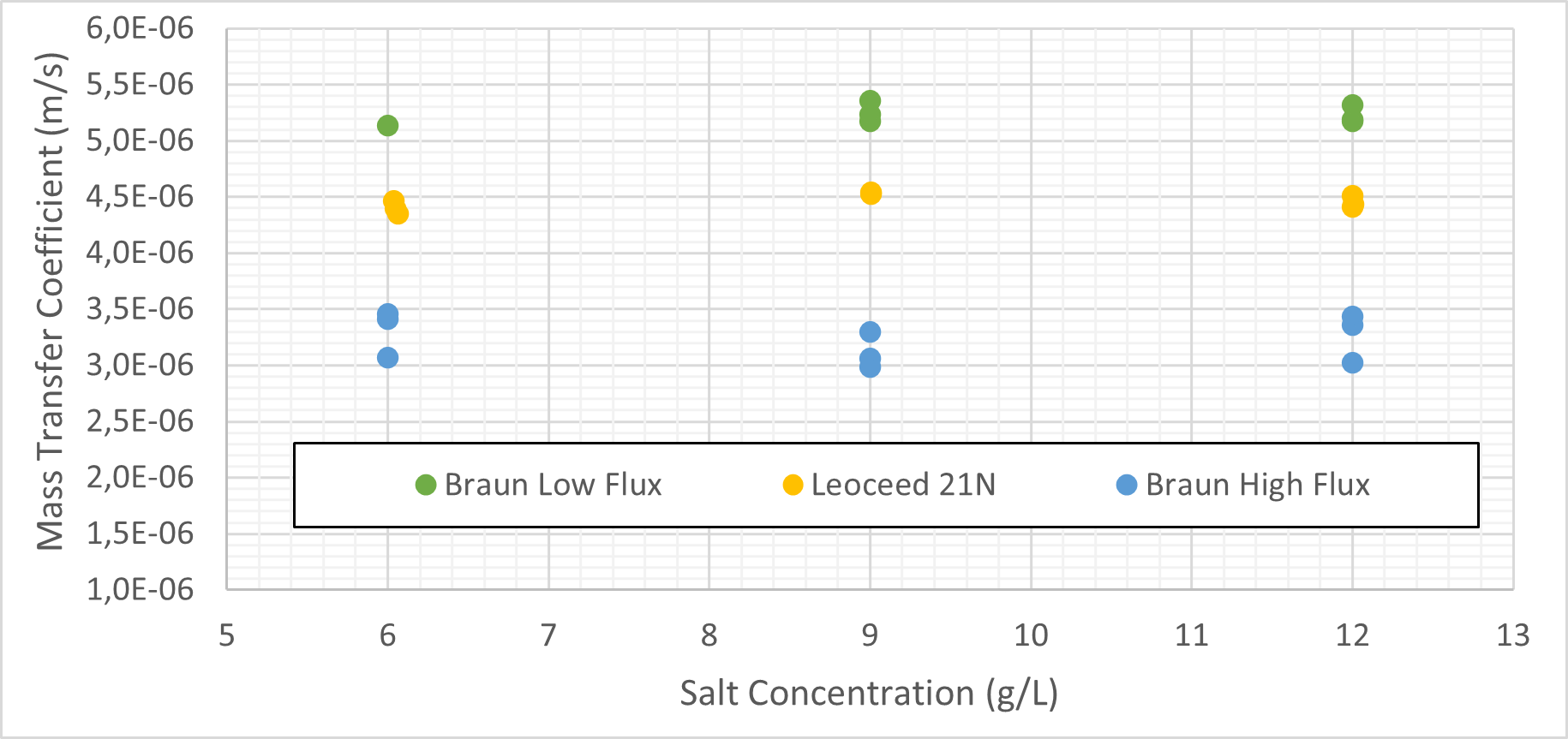
Figure 4 displays the salt mass transfer coefficient against the initial tube-side salt concentration. Table 6 gives the average coefficient as well as the Pearson correlation for each cartridge. The high flux cartridge mass transfer is virtually independent of initial salt concentration while the low flux cartridges show a slightly positive correlation.
During the use of the HFMD cartridge for respiratory support, consideration must be given to the blood composition as well as the gas transfer. The salt mass transfer coefficients determined above can give an indication of the requirements for clinicians to balance the solutes of interest as is done in regular dialysis.
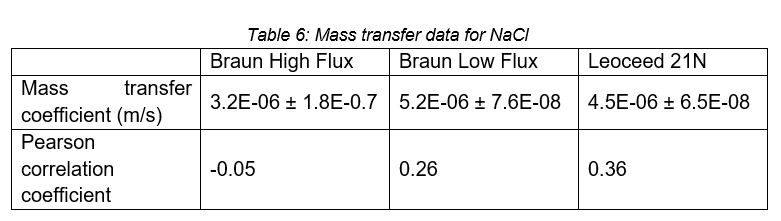
The first leakage test was to determine the amount of leakage from the inside of the fibres out to the cartridge shell. The results can be seen in Table 7.
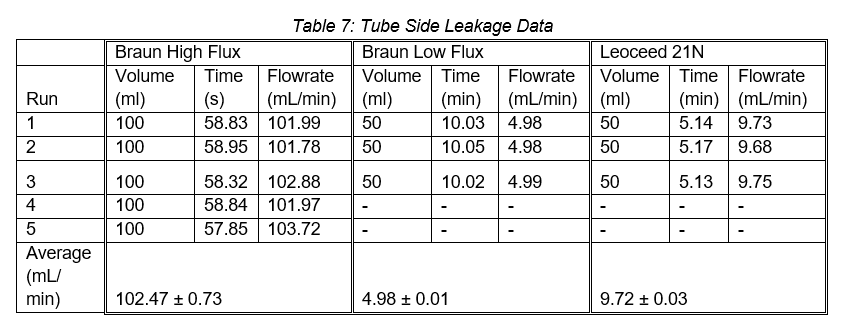
The second leakage test determined the leakage rate from the cartridge shell into the fibres. From this test, only the Leoceed 21N showed any leakage with a leakage rate of 6.10 ± 0.63mL/min across three runs.
The third leakage test determined the net leakage experienced by the tube-side during operation. The results can be seen in Table 8.
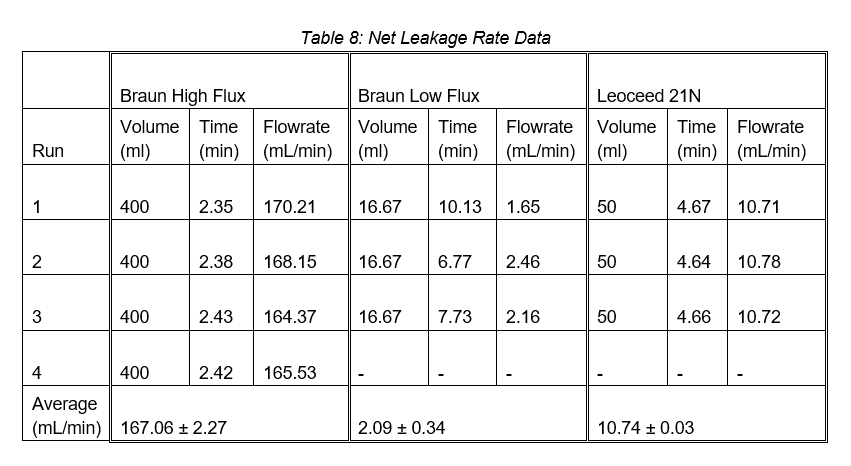
Apart from the Braun low flux cartridge, the net leakage across the membrane increased with the introduction of the shell-side liquid stream. This increase is likely due to a higher transmembrane pressure caused by the flow of the second stream, forcing more fluid through the membrane.
On average, human blood volume is 75ml/kg (Nadler et al., 1962), resulting in an average blood volume of 5 250ml for a 70kg human male. While side effects start appearing at around 15% blood loss, significant effects appear above 30%, and, above 40%, there is a substantial risk for organ failure, coma, and death (Johnson & Burns, 2021). With a net leakage rate of 167.06ml/min, this 40% threshold would be reached in around 12,5min. This makes the high flux cartridge unsuitable for use as a respiratory support device. The lower leakage rates of the other cartridges could be compensated for by blood or other fluid transfusions.
The high leakage rate of the high flux cartridge is likely due to its higher permeability to allow for larger molecules such as β2-microglobulin to pass through the membrane (Donadio et al., 2017). The leakage through all three cartridges could also be attributed to the differences between the water used in this work and the blood used in the usual dialysis procedure. The use of inadequate pumps with limited control could also have contributed as the flow rates and pressures could not be easily controlled. To fully assess the leakage risk, more sophisticated pumps, along with more representative fluids, will have to be employed. However, this study shows that high flux cartridges are not acceptable for use in a clinical setting.
The results of the sieving coefficient can be seen in Table 9.
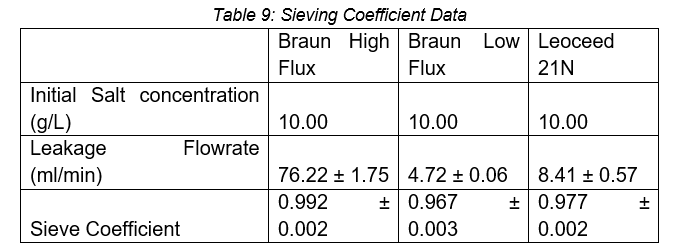
With a sieving coefficient close to one, all three cartridges display a nearly unrestricted transport of Na+ and Cl- across the membrane. This is as expected as these ions are small molecules (MW>500 Da) that can pass through both low and high flux membranes (Donadio et al., 2017). The high flux cartridge has the highest sieving coefficient due to its increased porosity and reliance on convective transport to allow for the removal of larger molecules (MW>500 Da) in addition to small molecules (Donadio et al., 2017).
If the HFMD is to be used for respiratory support in a liquid-liquid configuration, these molecules, and others like it, will have to be balanced by a physician. As mentioned, this is another advantage of the liquid-liquid configuration when compared to the liquid-gas configuration where a balancing medium is absent (L. Xu, 2020).
The global COVID-19 pandemic has shown the need for and importance of proper planning and resource management of healthcare resources. In this study, we have shown qualitatively that HFMD cartridges can remove carbon dioxide when using carbonated water in a liquid-liquid configuration. The use of high flux cartridges is not recommended as it has severe leakage effects that will require significant intervention to prevent harm to the patient. The remaining two cartridges were both able to achieve a substantial portion of the recommended carbon dioxide removal rate of 200ml/min. The Braun low flux performed the best with 75.8% removal, while the Leoceed 21N removed 64.4% of the required amount. These results are more than twice that found in previous research using a liquid-gas configuration under the same conditions of flowrate and pH. This not only proves that liquid-liquid HFMD should be considered for respiratory support but that the liquid-liquid configuration should be investigated as a possible improvement in the established ECMO field. Additionally, all cartridges were found to easily transport salt ions, confirming that a clinician will have to monitor and balance the blood solutes to prevent harm to the patient, as is done in conventional dialysis.
The main outcome of the study is confirmation that gas transfer does take place in the liquid-liquid configuration, with the unexpected finding that the transfer of CO2 is more rapid in the liquid-liquid configuration. This result is of great potential interest to the general operation of ECMO, but it is not certain to extend to the transfer of oxygen. It is also uncertain if the results are applicable when blood and an oxygen carrier are used as the two liquid streams.
Therefore, the findings here serve mainly as an indication that further research is merited to investigate the feasibility of liquid-liquid ECMO:
The authors acknowledge Dr. Neil Stacey for his assistance with the laboratory work, merSETA for funding, as well as the University of Witwatersrand for the use of their laboratories.
The authors have no conflicts of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent statement/Ethical approval: Not required.
Arthurs, G., & Sudhakar, M. (2005). Carbon dioxide transport. Continuing Education in Anaesthesia Critical Care & Pain, 5(6), 207–210. doi: 10.1093/BJACEACCP/MKI050
Asahi Kasei Medical. (2012). Leoceed series | Asahi Kasei Medical Co., Ltd. Retrieved 1 December 2020, from http://www.gml-dialyza.cz/downloads/products/Leoceed.pdf
Braun, B. (2021). Product Range of Dialysis disposables. Retrieved 29 September 2020, from https://avitum.com.pl/_files/ulotki_oferta_handlowa/materialydo- dializy.pdf
Baker, R. W., & Low, B. T. (2015). Membrane Separation. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. doi: 10.1016/B978-0- 12-409547-2.11674-9
Boyd, C. E. (2015). pH, Carbon Dioxide, and Alkalinity. In Water Quality (pp. 153–178). Springer, Cham. doi: 10.1007/978-3-319-17446-4_8
Carroll, D. (2021). A breath of fresh perfluorocarbon. Retrieved 16 November 2021, from https://engineering.cmu.edu/news-events/news/2017/11/20-nelson-perfluorocarbon.html
Claar, D. D., & Hyzy, R. C. (2017). Refractory Hypoxemia and Acute Respiratory Distress Syndrome Adjunctive Therapies: An Open Question? Annals of the American Thoracic Society, 14(12), 1768–1769. doi: 10.1513/ANNALSATS.201707-547ED
Donadio, C., Kanaki, A., Sami, N., & Tognotti, D. (2017). High-Flux Dialysis: Clinical, Biochemical, and Proteomic Comparison with Low-Flux Dialysis and On-Line Hemodiafiltration. Blood Purification, 44(2), 129–139. doi: 10.1159/000476053
Dondorp, A. M., Hayat, M., Aryal, D., Beane, A., & Schultz, M. J. (2020). Respiratory Support in COVID-19 Patients, with a Focus on Resource-Limited Settings. The American Journal of Tropical Medicine and Hygiene, 102(6), 1191. doi: 10.4269/AJTMH.20-0283
Shekar, K. (2022). Extracorporeal Life Support Organisation COVID-19 Interim Guidelines [Ebook]. Elso. Retrieved from https://www.elso.org/Portals/0/Files/pdf/ELSO%20covid%20guidelines%20final.pdf
ELSO. (2021). ELSO Center Directory | Extracorporeal Membrane Oxygenation | ECLS. Retrieved 7 November 2021, from https://www.elso.org/Membership/CenterDirectory.aspx. Retrieved: 1 April 2022
Espinoza-Gómez, H., & Lin, S. W. (2003). Development of Hydrophilic Ultrafiltration Membrane from Polysulfone-Polyvinylpyrrolidone. Revista de La Sociedad Química de México, 47(1), 53–57. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0583- 76932003000100008&lng=es&nrm=iso&tlng=en
Evseev, A. K., Zhuravel, S. V., Alentiev, A. Y., Goroncharovskaya, I. V., & Petrikov, S. S. (2019). Membranes in Extracorporeal Blood Oxygenation Technology. Membranes and Membrane Technologies, 1(4), 201–211. doi: 10.1134/s2517751619040024
Fasano, A., & Sequeira, A. (2017). Extracorporeal Blood Oxygenation. Modeling, Simulation and Applications, 18, 205–226. doi: 10.1007/978-3-319-60513- 5_5
Gattinoni, L., Vassalli, F., Romitti, F., Vasques, F., Pasticci, I., Duscio, E., & Quintel, M. (2019). Extracorporeal gas exchange: When to start and how to end? Critical Care, 23(Suppl 1), 1–7. doi: 10.1186/s13054-019-2437-2
Gimbel, A. A., Flores, E., Koo, A., García-Cardeña, G., & Borenstein, J. T. (2016). Development of a biomimetic microfluidic oxygen transfer device. Lab on a Chip, 16(17), 3227–3234. doi: 10.1039/C6LC00641H
Gorman, E., Connolly, B., Couper, K., Perkins, G. D., & McAuley, D. F. (2021). Non-invasive respiratory support strategies in COVID-19. The Lancet Respiratory Medicine, 9(6), 553–556. doi: 10.1016/S2213-2600(21)00168-5
Hlastala, M. P., & Souders, J. E. (2012). Perfluorocarbon Enhanced Gas Exchange. American Journal of Respiratory and Critical Care Medicine, 164(1), 1–2. doi: 10.1164/AJRCCM.164.1.2104021A
Hojyo, S., Uchida, M., Tanaka, K., Hasebe, R., Tanaka, Y., Murakami, M., & Hirano, T. (2020). How COVID-19 induces cytokine storm with high mortality. Inflammation and Regeneration, 40(1). doi: 10.1186/S41232-020-00146-3
Jägers, J., Wrobeln, A., & Ferenz, K. B. (2020). Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflügers Archiv - European Journal of Physiology 2020 473:2, 473(2), 139–150. doi: 10.1007/S00424-020-02482-2
Johnson, A. B., & Burns, B. (2021). Hemorrhage. Complications in Small Animal Surgery, 72–78. https://www.ncbi.nlm.nih.gov/books/NBK542273/
Kandler, M. A., Von der Hardt, K., Schoof, E., Dötsch, J., & Rascher, W. (2001). Persistent improvement of gas exchange and lung mechanics by aerosolized perfluorocarbon. American Journal of Respiratory and Critical Care Medicine, 164(1), 31–35. doi: 10.1164/ajrccm.164.1.2010049
Kashani, J., Shih, R. D., Cogbill, T. H., Jang, D. H., Nelson, L. S., Levy, M. M., Parker, M. M., Castanares-Zapatero, D., Laterre, P.-F., Castanares-Zapatero, D., Laterre, P.-F., Barney, J. B., Abraham, E., Murugan, R., Kellum, J. A., Sonneville, R., Ely, E. W., Sharshar, T., Maharaj, D., … Sun, B. (2012). Sieving Coefficient. Encyclopedia of Intensive Care Medicine, 2077–2077. doi: 10.1007/978-3-642-00418- 6_3300
Khandpur, R. S. (2020). Hollow Fibre Dialyser. Compendium of Biomedical Instrumentation, 965–967. doi: 10.1002/9781119288190.CH178
Lyons, C., & Callaghan, M. (2020). The use of high-flow nasal oxygen in COVID-19. Anaesthesia, 75(7), 843–847. doi: 10.1111/anae.15073
Machalinski, A. (n.d.). Hypovolemic Shock: Symptoms, Stages, Causes, Diagnosis, and Treatment. Retrieved October 25, 2021, from https://www.webmd.com/a-to-z-guides/hypovolemic-shock
Maclaren, G., Fisher, D., & Brodie, D. (2020). Preparing for the Most Critically Ill Patients with COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA - Journal of the American Medical Association, 323(13), 1245– 1246. doi: 10.1001/jama.2020.2342
Makdisi, G., & Wang, I. (2015). Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. Journal of Thoracic Disease, 7(7), E166– E176. doi: 10.3978/J.ISSN.2072-1439.2015.07.17
Martin, D. S., & Grocott, M. P. W. (2013). Oxygen therapy in critical illness: Precise control of arterial oxygenation and permissive hypoxemia. Critical Care Medicine, 41(2), 423–432. doi: 10.1097/CCM.0B013E31826A44F6
Matthay, M. A., Aldrich, J. M., & Gotts, J. E. (2020). Treatment for severe acute respiratory distress syndrome from COVID-19. The Lancet Respiratory Medicine, 8(5), 433–434. doi: 10.1016/S2213-2600(20)30127-2
Mayo Clinic. (2020). ARDS - Symptoms and causes. Retrieved 6 October 2021, from https://www.mayoclinic.org/diseases-conditions/ards/symptoms-causes/syc- 20355576
Messina, Z., & Patrick, H. (2021). Partial Pressure of Carbon Dioxide. StatPearls Publishing.
Mirco, N., Andrea, C., Angelo, G., Brambillasca, P., Federico, L., Michele, P., Giuseppe, G., Daniele, B., Francesco, F., Richard, N., Luca, L., Maurizio, C., & Carlo, M. (2020). At the Epicenter of the Covid-19 Pandemic and Humanitarian Crises in Italy: Changing Perspectives on Preparation and Mitigation. Catalyst: Innovations in Care Delivery, Figure 1, 1–5. doi: 10.1056/CAT.20.0080
Mishra, V., Svennevig, J., Bugge, J., Andresen, S., Mathisen, A., Karlsen, H., Khushi, I., & Hagen, T. (2010). Cost of extracorporeal membrane oxygenation: evidence from the Rikshospitalet University Hospital, Oslo, Norway. European Journal of Cardio-Thoracic Surgery : Official Journal of the European Association for Cardio- Thoracic Surgery, 37(2), 339–342. doi: 10.1016/J.EJCTS.2009.06.059
Munshi, L., & Hall, J. B. (2021). Respiratory Support During the COVID-19 Pandemic: Is It Time to Consider Using a Helmet? JAMA, 325(17), 1723–1725. doi: 10.1001/JAMA.2021.4975
Nadler, S. B., Hidalgo, J. U., & Bloch, T. (1962). Prediction of blood volume in normal human adults. Surgery, 51(2), 224–232. doi: 10.5555/URI:PII:0039606062901666
Neri, M., Villa, G., Garzotto, F., Bagshaw, S., Bellomo, R., Cerda, J., Ferrari, F., Guggia, S., Joannidis, M., Kellum, J., Kim, J. C., Mehta, R. L., Ricci, Z., Trevisani, A., Marafon, S., Clark, W. R., Vincent, J.-L., Ronco, C., & alliance, on behalf of the N. S. I. (NSI). (2016). Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Critical Care, 20(1). doi: 10.1186/S13054-016-1489-9
Park, M., Costa, E. L. V., Maciel, A. T., Silva, D. P. e, Friedrich, N., Barbosa, E. V. S., Hirota, A. S., Schettino, G., & Azevedo, L. C. P. (2013). Determinants of Oxygen and Carbon Dioxide Transfer during Extracorporeal Membrane Oxygenation in an Experimental Model of Multiple Organ Dysfunction Syndrome. PLoS ONE, 8(1), 54954. doi: 10.1371/JOURNAL.PONE.0054954
Pfeifer, M., Ewig, S., Voshaar, T., Randerath, W. J., Bauer, T., Geiseler, J., Dellweg, D., Westhoff, M., Windisch, W., Schönhofer, B., Kluge, S., & Lepper, P. M. (2020). Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration, 99(6), 521–542. doi: 10.1159/000509104
Pittman, R. N. (2011). Chapter 4: Oxygen Transport. In Regulation of Tissue Oxygenation. Morgan & Claypool Life Sciences.
Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R., & Salem, R. (2020). The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology, 0, 1446. doi: 10.3389/FIMMU.2020.01446
Ramanathan, K., Antognini, D., Combes, A., Paden, M., Zakhary, B., Ogino, M., MacLaren, G., Brodie, D., & Shekar, K. (2020). Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. The Lancet. Respiratory Medicine, 8(5), 518. doi: 10.1016/S2213-2600(20)30121-1
Riess, J. G. (2009). Understanding the Fundamentals of Perfluorocarbons and Perfluorocarbon Emulsions Relevant to In Vivo Oxygen Delivery. Artificial Cells, Blood Substitutes, and Biotechnology, 33(1), 47–63. doi: 10.1081/BIO-200046659
Rubin, D. M., Stacey, N. T., Matambo, T., & Hildebrandt, D. (2020). Oxygen transfer characteristics of a hollow fiber dialyser: toward possible repurposing of dialysers as blood oxygenators in the context of constrained availability of respiratory support. MedRxiv, Xx, 2020.04.06.20055236. https://www.medrxiv.org/content/10.1101/2020.04.06.20055236v2%0Ahttps://www.m edrxiv.org/content/10.1101/2020.04.06.20055236v2.abstract
Rubin, D. M., Stacey, N. T., Matambo, T., Vale, C. Do, Sussman, M. J., Snyman, T., Mer, M., & Hildebrandt, D. (2020). Toward Respiratory Support of Critically Ill COVID-19 Patients Using Repurposed Kidney Hollow Fiber Membrane Dialysers to Oxygenate the Blood. Journal of Healthcare Engineering, 2020. doi: 10.1155/2020/8862645
Sidebotham, D., Allen, S. J., McGeorge, A., Ibbott, N., & Willcox, T. (2012). Venovenous Extracorporeal Membrane Oxygenation in Adults: Practical Aspects of Circuits, Cannulae, and Procedures. Journal of Cardiothoracic and Vascular Anesthesia, 26(5), 893–909. doi: 10.1053/J.JVCA.2012.02.001
Sumin, L., Youguang, M., Shuhua, S., & Chunying, Z. (2010). The effect of hydrophobic modification of zeolites on CO2 absorption in different solvents. Brazilian Journal of Chemical Engineering, 27(2), 327–338. doi: 10.1590/S0104- 66322010000200011
Takahashi, N., Nakada, T., & Oda, S. (2018). Efficient CO2 removal using extracorporeal lung and renal assist device. Journal of Artificial Organs 2018 21:4, 21(4), 427–434. doi: 10.1007/S10047-018-1058-X
Tange, Y., Migita, H., Yoshitake, S., Isakozawa, Y., Takesawa, S., Imamiya, T., & Yoshida, T. (2012). Dialysate with High Partial Pressure of Oxygen Enhances Oxygenation in Blood during Simulated Circulation of Hemodialysis. Advanced Biomedical Engineering, 1(0), 43–46. doi: 10.14326/ABE.1.43
Tange, Y., & Yoshitake, S. (2019). Oxygen delivery to the blood stream by the dialysis fluid during continuous renal replacement therapy ex vivo. Journal of Artificial Organs 2019 22:2, 22(2), 104–109. doi: 10.1007/S10047-018-1085-7
Tawfic, Q. A., & Kausalya, R. (2011). Liquid Ventilation. Oman Medical Journal, 26(1), 4. doi: 10.5001/OMJ.2011.02
ter Beek, O. E. M., Pavlenko, D., & Stamatialis, D. (2020). Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkinTM polymer blends. Journal of Membrane Science, 604, 118068. doi: 10.1016/J.MEMSCI.2020.118068
Xu, H., Li, Y., Gao, W., Zhang, H., & Xu, L.-X. (2011). Hyperoxygenated Solutions in Clinical Practice: Preventing or Reducing Hypoxic Injury: Journal of International Medical Research, 39(5), 1589–1606. doi: 10.1177/147323001103900502
Xu, L. (2020). Hollow Fiber Membrane Dialysers: Gas transfer properties of Hollow Fibre Membrane Dialysers repurposed for respiratory support in critically ill COVID-19 patients. doi: 10.13140/RG.2.2.33285.83680
Yang, W., Hong, J., Zeng, Q., Tao, J., Chen, F., Dang, R., Liang, Y., Wu, Z., & Yang, Y. (2016). Improvement of Oxygenation in Severe Acute Respiratory Distress Syndrome With High-Volume Continuous Veno-venous Hemofiltration: Global Pediatric Health, 3, 2333794X1664569. doi: 10.1177/2333794X16645699
Yeager, T., & Roy, S. (2017). Evolution of Gas Permeable Membranes for Extracorporeal Membrane Oxygenation. Artificial Organs, 41(8), 700–709. doi: 10.1111/aor.12835