





Joel Philpott, Sudesh Sivarasu
ORCID ID: 0000-0003-4037-9193, 0000-0002-0812-568X
Division of Biomedical Engineering
Department of Human Biology
University of Cape Town
Western Cape
South Africa
Richard Raine
ORCID ID: 0000-0003-2075-7396
Division of Pulmonology
Department of Medicine
University of Cape Town
Western Cape
South Africa
Many diseases affect the efficacy of the respiratory system, and thus ventilatory support is required to improve the patient’s prognosis. One of the methods of providing this support is by using non-invasive, positive pressure, BiPAP ventilation. The purpose of this project is to develop a prototype of a medical device that can provide this support. This is achieved by understanding the requirements of the device, generating concepts that can satisfy these requirements, selecting a concept and constructing it. The selected concept makes use of two blower fans, the first to provide the inspiratory pressure support, and the second to provide the expiratory pressure support. The pressure delivered to the patient is controlled using an axial solenoid valve and a one-way valve. A PID control loop is then used to control the speed of the fans. The system developed is not able to meet all the requirements but would be able to do so with slight improvements. These improvements include redesigning the axial solenoid valve to reduce the pressure drop across the valve, redesigning the flow path of the circuit to reduce the pressure drop in sharp corners, and changing the inspiratory fan control loop implementation strategy. The flow rate sensor also needs to be improved to allow the device to perform accurate volume-controlled ventilation.
Keywords: BiPAP, non-invasive, ventilation, pressure controlled, flow control valve, flow rate sensor
| NPV | Negative Pressure Ventilation |
| PPV | Positive Pressure Ventilation |
| BiPAP | Bilevel Positive Airway Pressure |
| CPAP | Continuous Positive Airway Pressure |
| IPAP | Inspiratory Positive Airway Pressure |
| PEEP | Positive End Expiratory Pressure |
| WHO | World Health Organisation |
| PID | Proportional Integrative Derivative |
| I: E | Inspiratory: Expiratory |
Many diseases have harmful impacts on patients’ lungs, resulting in reduced blood oxygen levels and ultimately respiratory failure (Patel, 2020). To improve their prognosis, it is essential to provide support to the patient's respiratory system before it fails (Shelly & Nightingale, 1999). This support can either be invasive or noninvasive. Invasive therapy refers to mechanical ventilation administered via an endotracheal tube (a tube through the mouth into the throat near the larynx) or a tracheostomy tube (a tube through an incision in the trachea) (Shelly & Nightingale, 1999). Non-invasive therapy refers to respiratory therapy applied via a face mask.
Non-invasive therapy can be further split into two categories: Negative Pressure Ventilation (NPV) and Positive Pressure Ventilation (PPV). Negative pressure ventilation is the application of negative pressure to the outside of the thoracic region, assisting in opening the patient’s airways (Marcotte, 2020). This ventilation method is, however, not effective when the patient’s lung compliance is reduced, and the devices are typically cumbersome to use (Grasso, et al., 2008). PPV is the application of positive pressure inside the patient's lungs. This is achieved by blowing air into the patient's lungs through a mask, thereby preventing their lungs from collapsing.
Non-invasive PPV can be achieved through two different strategies: Continuous Positive Airway Pressure (CPAP) and Bilevel (also known as Bi-phasic) Positive Airway Pressure (BiPAP). CPAP provides constant pressure to the patient, regardless of whether they are inhaling or exhaling (Sharma, et al., 2011). BiPAP, on the other hand, provides two different pressure levels: a lower pressure when the patient is exhaling, and higher pressure when they are inhaling. The higher pressure is the Inspiratory Positive Airway Pressure (IPAP), and the lower pressure is the Positive End Expiratory Pressure (PEEP). BiPAP ventilation is more comfortable than CPAP ventilation, especially at higher pressures, and is therefore used more frequently to treat severe cases of respiratory distress (Patil, 2020).
The COVID-19 pandemic has placed significant strain on the healthcare systems in many countries, leaving patients untreated, or inadequately treated (Menon & Padhy, 2020). This project, therefore, aims to develop a non-invasive BiPAP ventilator that can be used in the treatment of patients infected with the SARS-COV2 virus. The device differs from conventional ventilators by changing the pneumatic circuit. Conventional ventilators use a single fan module and control the speed of the fan to control the pressure output. This device uses two fans, with one fan controlling the expiratory pressure, and the second controlling the inspiratory pressure. The fans do not need to change speed rapidly, allowing for more accurate control of the pressure levels. The following research question is therefore addressed in this article: Can the use of a dual fan pneumatic circuit combined with the use of an axial solenoid valve reduce the reliance on the change of speed of the fan to produce BiPAP ventilation?
Firstly, the required performance characteristics of the device are identified. These will ensure the device developed meets the market standards in terms of performance as well as the requirements of the World Health Organisation (WHO). To limit the scope of the project, and because the device developed is only a prototype, some of the requirements are excluded. The requirements are shown in Table 1.

While considering the intellectual property landscape, different concept generation strategies are used to develop solutions that would satisfy the requirements above. These concepts are then screened by their functional performance, as well as regulatory and reimbursement considerations. The resulting pneumatic circuit of the device is shown in Figure 1 below.

All components are purchased as off-the-shelf parts except the flow rate sensor and the control valve 1. The design of these components is discussed below.
Flowrate sensors are typically expensive components, so to reduce the cost of the device, a flowrate sensor that makes use of the Venturi effect is designed. This relies on the principle that a fluid flowing creates a negative pressure next to it, and the faster the fluid is flowing, the greater the negative pressure. If the diameter of a pipe is reduced, the velocity of the fluid flowing through the pipe changes, thus causing a pressure differential. The flow rate can be calculated using Bernoulli's equation shown below (Muson, Young, & T.H., 1994).
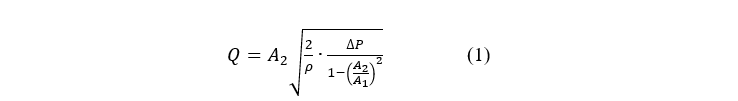
In the equation, A2 represents the smaller area of the pipe, A1 the larger area of the pipe, the density of the air, and Q the flowrate. By iterating through different differential pressure sensors and optimising the design for the most accurate flow rate reading, it is calculated that the ideal diameters for A1 and A2 are 20mm and 7.5mm respectively. The design is 3D printed, and the inner area is drilled and reamed to ensure dimensional accuracy. The assembled flow sensor is shown in Figure 2.

The control valve is required to open when the patient inhales, and close when they exhale. It must therefore close quickly, and seal well to ensure the correct pressure is applied to their lungs. Various design iterations are built and tested, and the final iteration is shown in Figure 3.

This design makes use of a movable plunger in the flow path to open and close the valve. When the solenoid coil around the outside is not activated, the air pressure, as well as the spring, pushes the plunger to the right, sealing it against the casing. When the solenoid coil is activated by applying a voltage to it, the magnetic field of the solenoid and the permanent magnets on the plunger interact, pushing the plunger to the left, up the flow path. This allows air to flow through the valve and to the patient. The air passes between the magnets and the coil, so it does not need to go through any sharp corners, thus reducing the pressure drop across the valve.
The purchased and manufactured components are assembled, using 3D printed parts to connect all the components. All the connections are designed to pressfit together, and tape is used where necessary to seal the connections. The resulting device is shown in Figure 4.

The four potentiometers on the front face of the device are used to control the breathing rate, PEEP pressure, IPAP pressure, and I:E ratio. Veroboard is also used to make all the required electronic connections and ensure that the connections do not loosen during operation.
One of the key components of the device is the algorithm used to control the pressure delivered to the patient. To identify what algorithm would be suitable, a test is done on the system without any feedback. The IPAP and PEEP fans are set at a constant speed and the control valve is controlled with a square wave. The results of this test are shown in Figure 5.

The P1 reading is taken just after the PEEP fan, the P3 reading is taken just after the IPAP fan, and the P5 reading is taken at the patient end. It is noted that the P1 readings do not change when the control valve opens and closes. A continuous Proportional Integrative Derivative (PID) control loop can therefore be run on the value from P1. It is also noted that the P3 reading drops significantly when the valve opens and rises again when the valve closes. Because of this, a PID loop is implemented for the second half of the inspiration phase. The pressure readings are therefore allowed to settle to a relatively constant value before the speed of the fan is changed.
The design is tested using a passive test lung with a volume of 1.0 l, and compliance of 20ml/cmH2O, in the place of a patient. The first characteristic of the ventilator tested is the flow rate delivered to the patient. The results of this test are shown in Figure 6.

The device achieves a maximum flow rate of 103.81 l/min, which is less than the 120 l/min requirement. During component testing, it is found that the fans can produce the required flow rate, so the flow path of the air needs to be optimised to increase the flow rate. This can be achieved by improving the design of the flow control valve and reducing the pressure drop across the valve. This change would also increase the maximum pressure delivered to the patient. Figure 7 shows the maximum pressure delivered to the patient during the inspiratory phase. The maximum inspiratory pressure is measured to be 33.45 cmH2O, less than the 35 cmH2O requirement.

One of the issues with the control algorithm that became clear during testing is that as the time of the inspiratory phase decreases, so does the time that the PID loop is run for the IPAP fan. This results in an unstable loop, where the speed of the IPAP fan and thus the IPAP pressure delivered to the patient oscillates between breaths. This is seen in the test with an Inspiratory: Expiratory (I:E) ratio of 1:6 (inhalation is one-seventh of the total cycle). The results of this test are shown in Figure 8.

The time that the PID loop is used is indicated by the dark blue line labelled "PID Loop". For an I:E ratio of 1:6, the IPAP PID loop is only used for 7.1% of the cycle. The resulting oscillation of the fan speed is seen in the P3 readings, the pressure just after the IPAP fan. Similar oscillation in the IPAP fan speed is seen with an increased breathing rate (30 breaths per minute), as shown in Figure 9.

Lastly, the PEEP pressure is tested. The required maximum PEEP pressure is 25 cmH2O and the device can supply this pressure. The PEEP fan stabilises at a fixed speed for all the tests indicating the control algorithm for the PEEP fan works correctly.
The constructed device was able to meet some of the requirements, but not all of them. The flow control valve used in the device should be redesigned to reduce the pressure drop across the valve and increase the maximum flow rate. The control algorithm for the IPAP fan should also be improved to increase its stability. Improvements should be made to the flow rate sensor to allow accurate, volumecontrolled ventilation. Although it can provide basic pressure controlled non-invasive ventilation, further development is required for the device to have the same performance characteristics as conventional single fan ventilators.
The authors would like to acknowledge the contribution of colleagues at the University of Cape Town Medical Devices Lab, and staff assisting students in the lab for their assistance with advice and support during this study.
Grasso, F., Engelberts, D., Helm, E., Frndova, H., Jarvis, S., Talakoub, O., . . . Kavanagh, B. (2008). Negative-Pressure Ventilation. American Journal of Respiratory and Critical Care Medicine, 412-418. doi: 10.1164/rccm.200707-1004OC
James, F. K. (2019, May 17). healthline. Retrieved from What is Hypoxemia?: https://www.healthline.com/health/hypoxemia
Marcotte, B. (2020, April 17). Ventilators: Three centuries in the making. Retrieved from Medical Press: https://medicalxpress.com/news/2020-04-ventilators-centuries.html
Menon, V., & Padhy, S. (2020). Ethical dilemmas faced by health care workers during COVID-19 pandemic: Issues, implications and suggestions. Asian Journal of Psychiatry, 102-116. doi: 10.1016/j.ajp.2020.102116
MHRA. (2020, April 10). Rapidly Manufactured Ventilator System. Medicines & Healthcare Products Regulatory Agency.
Muson, B., Young, D., & T.H., O. (1994). Fundamentals of Fluid Mechanics, 2nd Ed. John Wilet and Sons, Inc.
Patel, B. K. (2020, April 24). Repiratory Failure. Retrieved from MSD Manual: https://www.msdmanuals.com/home/lung-and-airway-disorders/respiratory-failure-and-acute-respiratory-distress-syndrome/respiratory-failure
Patil, S. (2020). BiPAP vs CPAP in Treatment of OSAS. Chest, A2408-A2409. doi: 10.1016/j.chest.2020.09.008
SASA. (2020). Standards for Supply/Manufacture of Ventilators to South Africa During the COVID-19 Pandemic. South African Society of Anaesthesiologists.
Shaikh, J. (2020, October 15). What Are Blood Oxygen Levels? Chart. Retrieved from MedicineNet: https://www.medicinenet.com/what_are_blood_oxygen_levels/article.htm
Sharma, S., Agrawal, S., Damodaran, D., Sreenivas, V., Kadhiravan, T., Lakshmy, R., . . . Kumar, A. (2011). CPAP for the Metabolic Syndrome in Patients with Obstructive Sleep Apnea. New England Journal of Medicine, 2277-2286. doi: 10.1056/NEJMoA1103944
Shelly, M. P., & Nightingale, P. (1999). ABC of intensive care: Respiratory support. BMJ, 1674-1677. doi: 10.1136/bmj.318.7199.1674
WHO. (2020, April 15). World Health Organisation Institutional Repository for Information Sharing. Retrieved from Technical specifications for invasive and noninvasive ventilators for COVID-19: Interim guidance: https://apps.who.int/iris/handle/10665/331792