





Brandon Reabow, Sudesh Sivarasu
ORCID ID: 0000-0002-4534-2640, 0000-0002-0812-568X
Division of Biomedical Engineering
Department of Human Biology
University of Cape Town
Western Cape
South Africa
High Flow Nasal Oxygen (HFNO) medical devices are utilised to non-invasively oxygenate Coronavirus disease 2019 (COVID-19) and acute respiratory distress syndrome (ARDS) patients with mechanisms of clinical benefit. Most recently, HFNO has proven to be effective against hypoxemic symptoms by providing a range of oxygen concentration levels (FiO2) and aims to improve patient compliance and comfort with heating and humidification of inspiratory gases. HFNO has been shown in studies to be feasible in resource-constrained settings, relying on an affordable solution to drive an increased number of supplied devices to healthcare facilities to effectively treat patients independently of intensive care units (ICU). Therefore, a Proof of Concept was developed under an iterative design approach. A full cost summary of materials was R8 100, compared to at least R45 000 for current South African market competitors. Additionally, the solution was functionally tested to determine levels of verification in technical specifications and validation in addressing clinician and patient needs. The solution achieved associated 10 - 60 l/min flow rates; stable FiO2 from 50 - 100% (with a minimum of 40%); and inspiratory gas temperatures and humidity of up to 40 oC and 90% relative humidity (RH). Further design and development need to be conducted to output a high-performing and safe medical device as guided by ISO 80601-2-90.
Keywords: HFNO, COVID-19, ARDS, resource-constrained, low-cost, affordable, ICU, Proof of Concept, medical device
| FiO2 | - | Device oxygen and air concentration level |
| SpO2 | - | Patient oxygen saturation level |
| VFR | m3/S | Volumetric flow rate |
| C | - | Discharge coefficient for partial fluid viscosity |
| ΔΡ | Pa | Differential pressure |
| Α1 | m2 | Upstream venturi cross-sectional area |
| Α2 | m2 | Downstream venturi cross-sectional area |
| ρ | kg/m3 | Fluid Density |
| VFRoxygen | m3/s | Volumetric flow rate of oxygen gas |
| VFRair | m3/s | Volumetric flow rate of ambient air |
Patients with COVID-19 and ARDS experience symptoms related to respiratory failure. These include hypoxemic or low oxygen saturation levels (SpO2) and require intervention in oxygenation treatments. HFNO has been extensively used to treat COVID-19 patients, or patients with similar respiratory failure. The reason for this relates to the non-invasive characteristic of the treatment because patients do not need to be intubated, consequently improving chances for patients tolerating treatment without sedation.
Studies have shown that the use of HFNO mitigates risks involved in treating patients with more invasive mechanical ventilators, as associated mortality rates for these devices can be high. The use of HFNO to treat initial acute hypoxemic symptoms allows for patients to be weaned off ventilation treatments before reaching invasive mechanical ventilation (Calligaro et al., 2020).
HFNO has proven to be used in varying application settings. The use of HFNO within resource-constrained settings is feasible (Calligaro et al., 2020). HFNO may be used within ICU's and general pulmonology wards, and to the extent of home use (Calligaro et al., 2020; ResMed, 2021). This results in not requiring ICU facilities which reduces length of stay in ICU for ventilated patients and reduces staffing requirements without compromising patient care (Bice et al., 2013). Additionally, ICU and ventilator expertise are less of a requirement (Liu et al., 2021). Transportation of unstable patients is appropriate with HFNO treatments (Kedzierewicz et al., 2021). Moreover, high flow therapy is limited by the source of oxygen, whereby typical oxygen concentrators for home-use can produce up to 10 l/min (Hardavella et al., 2019). Therefore, hospital wall oxygen provides the most suitable approach for supplying HFNO devices with oxygen for meeting high flow rate benefits. Studies need to be conducted for HFNO treatment in pre-hospital settings but have been used as a first choice from home to emergency transport. These introduce exceeding battery energies associated with high energy consuming components for heating and humidification, as well as low oxygen delivery times with oxygen tanks like a 4.5-LME36 cylinder (Kedzierewicz et al., 2021).
HFNO is classified as non-invasive within a high flow nasal oxygen category. The treatment is aimed at providing high flow oxygen delivery with heated humidification. The effectiveness of the treatment is associated with the high-velocity gas expelling from the cannula of a patient interface. Therefore, the system is based off flow rate requirements with maximum flow rates generally delivered between 50 and 70 l/min (WHO, 2021). This provides the necessary airway pressures and advantageous PEEP effect without requiring sealing of a patient interface. Additionally, as enlisted by the World Health Organization (WHO), technical specifications may be adopted to provide a basis for the development of the HFNO proposed solution. (WHO, 2021)
HFNO has many components constituting to the device. These may be broken up into stages described as inspiratory gas flows through the device and consecutively includes: oxygen regulation and mixing, humidification and heating, and the patient interface stages. The oxygen regulation and mixing stage make use of active flow generation in types of air-oxygen blenders or built-in flow generators, whereas passive entrainment systems provide for an additional method of mixing. Oxygen/air mixtures can have a range of FiO2 from 0.2 to 1.0 and are usually dependent on input or generated flow rates (Nishimura, 2019; WHO, 2021). Dry gas, especially over longterm delivery, may cause mucociliary malfunction, epithelial damage, mucus plugging, ulceration of mucosa, and in this context, ventilator-induced lung injury (VILI) (Cerpa et al., 2015). Therefore, active heated humidifiers are used to primarily provide humidification at high flows. Additionally, a heated patient circuit is used to provide effective gas heating, as well as provide humidity regulation. Finally, a patient interface consisting of a nasal cannula provide an open system connection to the patient. Equipped with high flow rates, HFNO can provide more stable inspiratory flow rates and humidity, since low flow rate systems comparatively provide unmatched device settings due to inspiratory flow rates less than HFNO flow rates (Nishimura, 2019). Existing solutions for HFNO are internationally recognised, imported, and supplied in local supply chains. The cost range in the South African market is between R45 000 and R60 000 (High-Flow Nasal Cannula Oxygen COVID-19 cases, 2020). Therefore, the project aims to design and develop a high flow nasal oxygen device that is lowcost for improved accessibility and chance of local manufacturing to address the needsof resource-constrained and low-income settings for healthcare facilities.
Approaching a final concept with iterative design and development, the aim was to prototype an initial Proof of Concept with basic functionality that could be verifiedand validated. Therefore, the following requirements and their methods were considered throughout the project:
The final concept subsystem units are divided into the following: oxygen regulation and mixing unit, heating and humidification unit, and the patient interface. The final prototype is shown in Figure 1.
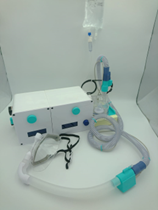
i. Oxygen regulation and mixing unit
The oxygen regulation and mixing unit incorporates a manual analog control solution whereby input oxygen and ambient air flow rates may be controlled in a lowcost manner. The unit utilises a Venturi entrainment device to entrain ambient air from the hospital wall oxygen outlet. Oxygen and air flow rates were determined by separate Venturi chambers that generate differential pressures across a decrease in diameters along the gas flow path. This differential pressure is measured, and Bernoulli's equation is used to calculate volumetric flow rates. The form of Bernoulli's equation
used is shown in Equation 1. With the user able to control flow rates of oxygen and air, the FiO2 is manually controlled. There is a relationship between these two flow rates that calculate the output FiO2, referred to in Equation 2 (Ely & Clapham, 2003). The nomenclature provides reference to the use and units of these equations.
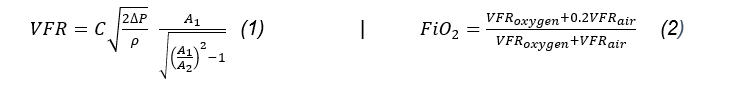
The testing of this unit involved the use of a portable tank compressor equipped with filters to provide compressed air at a maximum of 2.2 bars. The device would internally regulate the pressure between 0 and 4 bar, simulating the conditions from hospital oxygen wall supplies (SARAO, 2020). The MPX5100DP and the MPX5010DP differential pressure sensors were procured to provide analog measurement readings. These sensors are inexpensive, coupled with the Venturi chamber to produce affordable flow meters. Signal filtering techniques were used to mitigate noisy sensor measurements, particularly for differential pressure, volumetric flow rates, and calculated outputs which include total flow rate and FiO2. These techniques involved digital low-pass filtering, passive laminar inducing components, and running averages. The flow rates were verified with the highly accurate Sensirion SFM3000 flow meter in the UCT MDL. A basic Liquid Crystal Display (LCD) was used to present all required measurements and calculations. This allowed for accurate usability and display of measurements. Ultimately, results were captured for the controlled oxygen and entrained ambient air flow rates, as well as the calculated summated total flow rate and FiO2 percentages. A custom control valve for the entrained air was developed with six different settings: setting "0" referring to the least air entrained and setting "5" referring to the most air entrained. Therefore, a higher setting number will provide for a lower FiO2 output for a given oxygen flow rate input.
ii. Heating and humidification unit
The method to heat and provide humidity to the inspiratory gases requires active heater element components. The mechanical design was developed to transport gas from the oxygen regulation and mixing unit to the patient interface efficiently, with the procured water chamber by Inspira and heated breathing circuit by BMC. Two sensing units, at chamber-end and patient-end, were incorporated to measure relative humidity and temperature; and monitor effectiveness of active humidification of the water chamber and heating regulation of the breathing circuit. By applying air flow through the unit and allowing for the chamber water and heat exchanger to warm up, results show the characteristics of heating and humidification along the path to the patient. With the use of a basic control system, Pulse Width Modulated (PWM) triggers were utilised to control average voltages supplied to the water chamber and heated breathing circuit power elements. This will be controlled via a user menu implemented with a rotary encoder. Moreover, two DHT22 temperature and humidity sensors were used for chamber-end and patient-end measurements. Additionally, a temperature sensor is placed in the chamber heater element to prevent overheating. All voltages and sensor measurements are presented on an LCD as the unit's user interface.
iii. Patient interface
A patient interface was developed to deliver inspiratory gases through a nasal cannula. The nasal cannula provides high-velocity gas deliverance into the nostrils of a patient. Throughout testing, the patient interface was only demonstrated and not connected to any person with the device on. Nevertheless, the interface provides adjustable fitment around the face with comfortable padding to insulate frontal sinuses.Additionally, a removable face shield may be placed onto the interface to minimise aerosolization effects associated with HFNO, standard oxygen masks and nasal cannula; and limit infectious spreading of respiratory-related diseases (Li et al., 2020).
i. Oxygen regulation and mixing unit
The sensor measurements and calculations were captured from a single test whereby total flow rates were stepped in increasing 10l/min flow rates, for each entrained air control valve setting (0 - 5). Figure 2 and Figure 3 illustrate the dependency of total flow rates and FiO2 for an input oxygen flow rate and each entrainer valve setting.
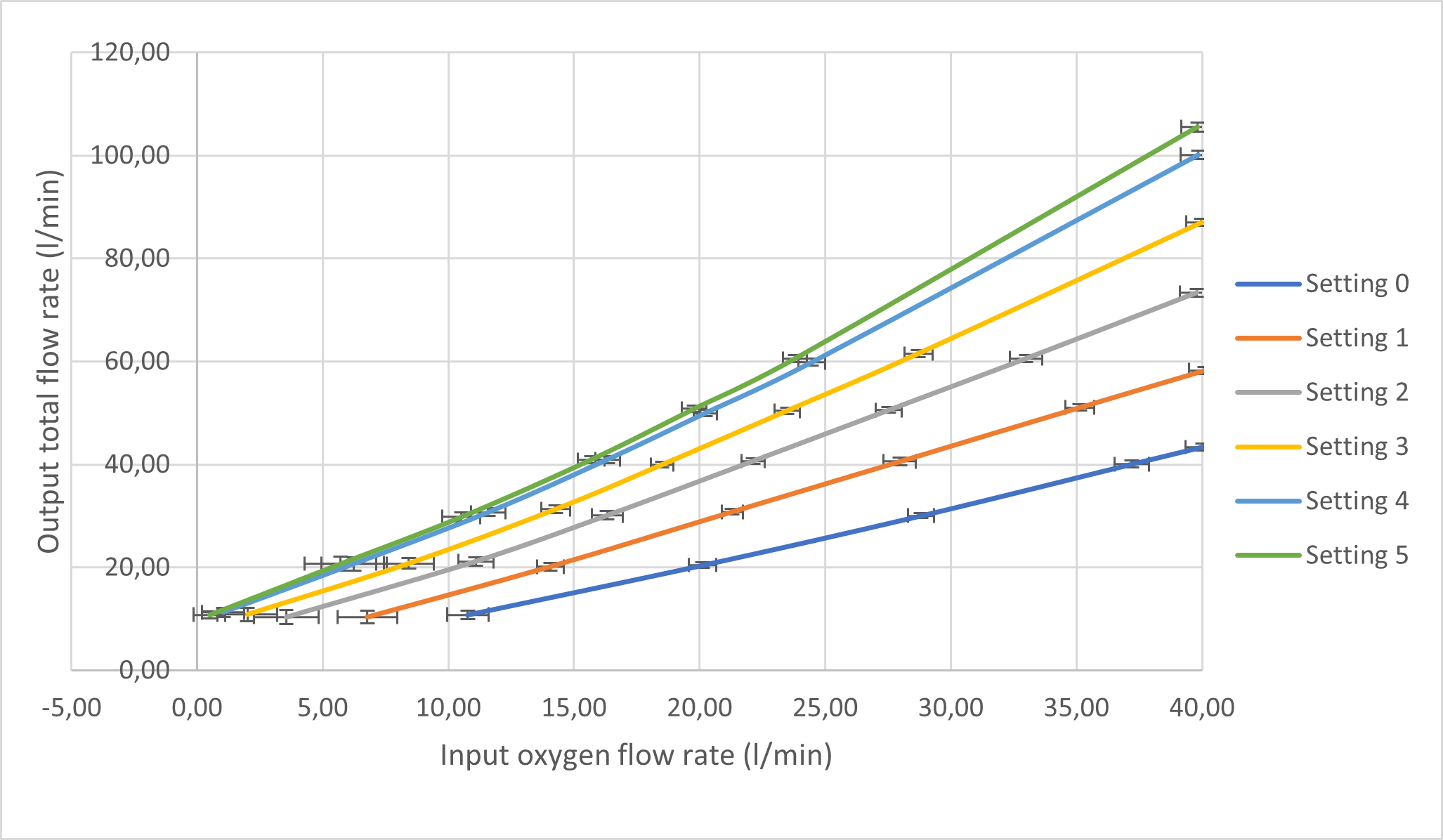
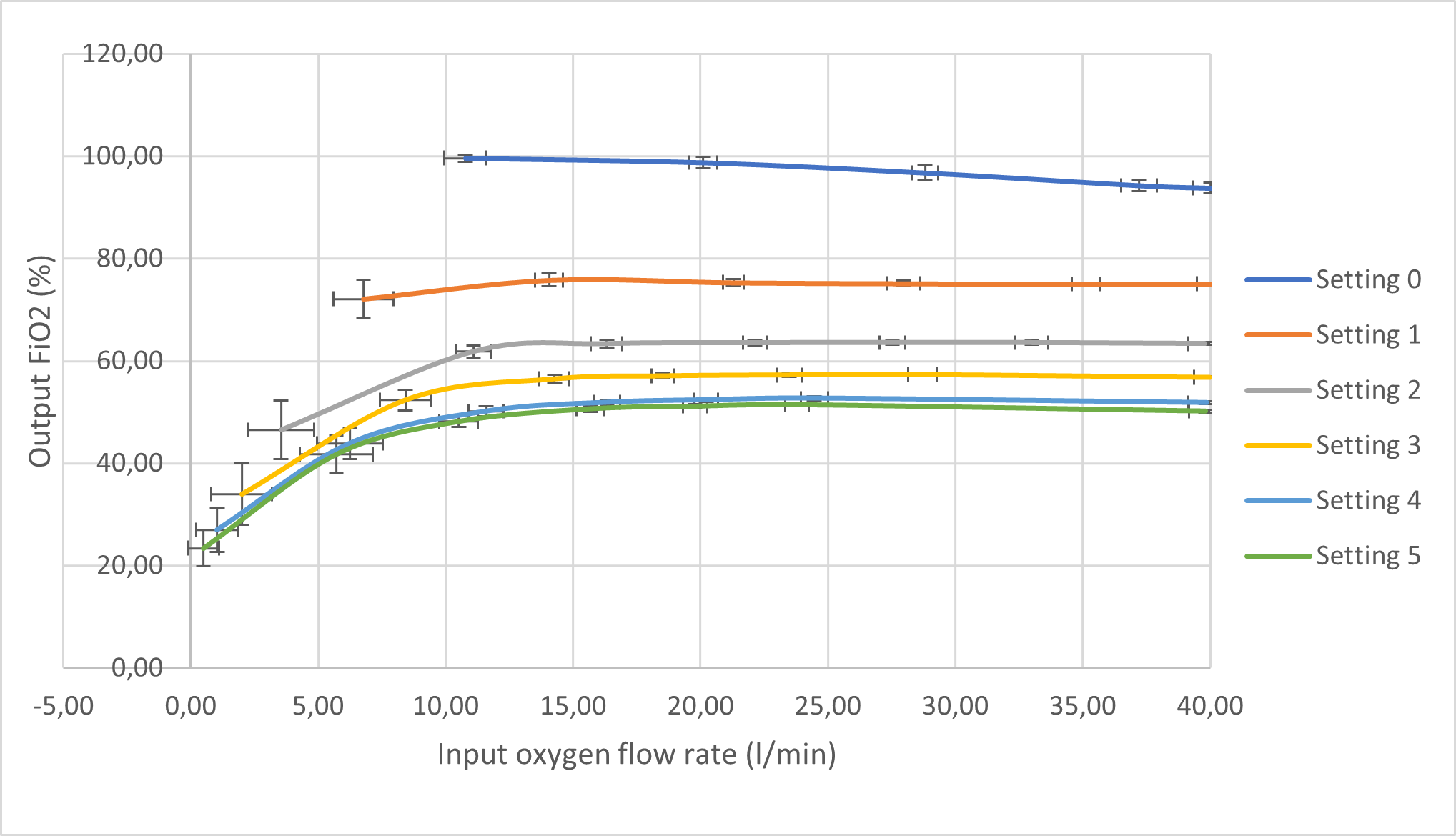
The following aspects of the results for the unit are discussed:
ii. Heating and humidification unit
Initial tests showed that it was beneficial to allow the water chamber to heat up before applying flow rates. The temperature read from the chamber temperature sensor displayed to settle at the point flow was introduced to the system. Therefore, preliminary tests at intermittent 30 l/min total flow were introduced throughout the entire device. The results are shown in Figure 4 with the following aspects to be discussed:
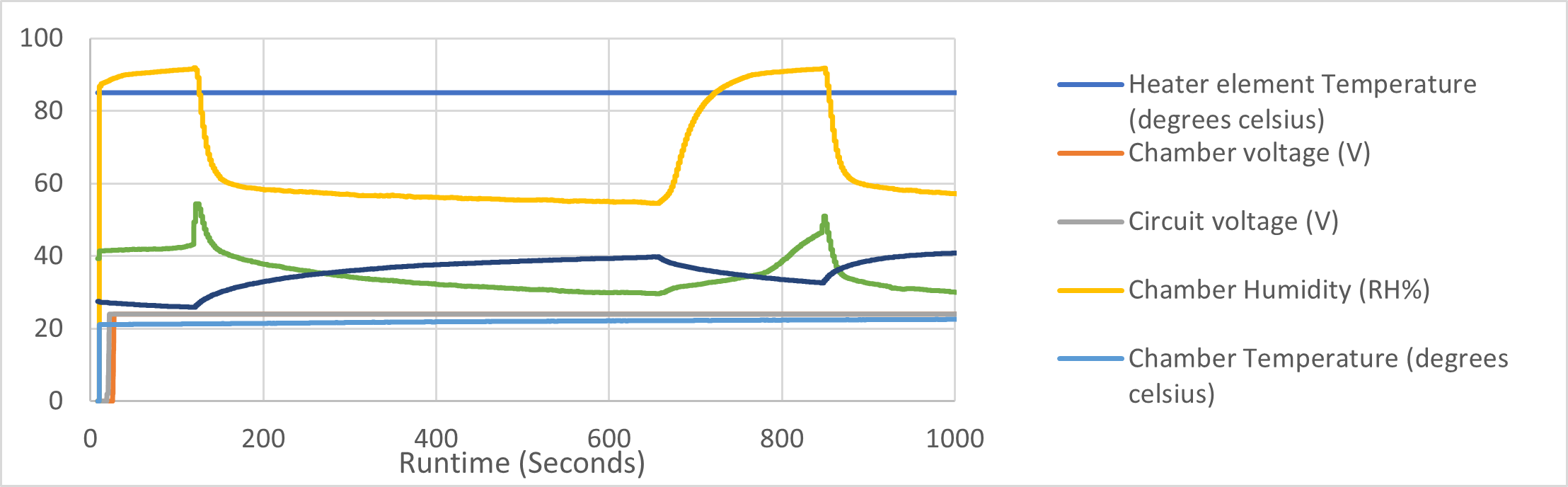
iii. Patient interface
The patient interface is demonstrated in Figure 5, depicting the fitment onto the patient's face, as well as the position and connections of all components relative to the patient.

iv. Validation discussion
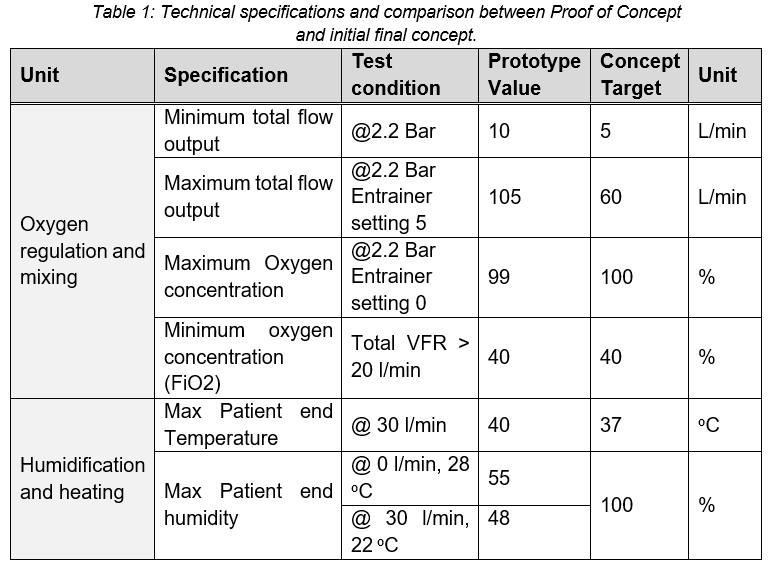
The general technical specifications for the target Proof of Concept (based on WHO technical specifications and similar device specifications) and the tested prototype is presented in Table 1.
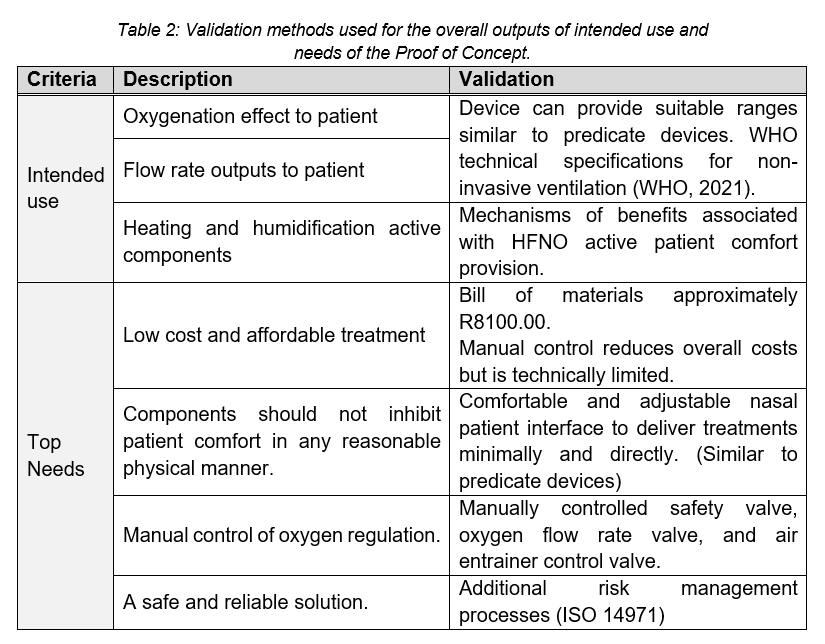
The prototype may be validated to the intended use of the device as well as the top needs defined. This is summarized in Table 2.
The project in its current state of development was able to show the feasibility of a low-cost and affordable High Flow Nasal Oxygen solution, both quantitatively and qualitatively. It is well equipped to meet the current requirements and needs of a resource-constrained setting, using a combination of manual analog and digital control. Further design and development processes should be considered to improve the solution's performance in terms of accuracy, usability, safety, and general quality management. Verification in terms of ISO 80601-2-90:2021 should be considered to achieve basic safety and performance (ISO, 2021). Further optimisation and refinement with standardised testing in the laboratory and clinical settings will be conducted.
The authors would like to acknowledge all enablers involved in collaboration, manufacturing processes, and testing equipment, particularly the UCT Mechanical Engineering technical staff, UCT MDL colleagues, and the UCT Private Hospital Technologists and Pulmonologists.
Bice, T., Cox, C., & Carson, S. (2013). Cost and Health Care Utilization in ARDS—Different from Other Critical Illness? Seminars in Respiratory and Critical Care Medicine, 34(04), 529-536.doi: 10.1055/s-0033-1351125
Calligaro, G. L., Lalla, U., Audley, G., Gina, P., Miller, M. G., Mendelson, M., Dlamini, S., Wasserman, S., Meintjes, G., Peter, J., Levin, D., Dave, J. A., Ntusi, N., Meier, S., Little, F., Moodley, D. L., Louw, E. H., Nortje, A., Parker, A., . . .Koegelenberg, C. F. N. (2020). The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine, 28, 100570.doi: 10.1016/j.eclinm.2020.100570
Cerpa, F., Cáceres, D., Romero-Dapueto, C., Giugliano-Jaramillo, C., Pérez, R., Budini, H., Hidalgo, V., Gutiérrez, T., Molina, J., & Keymer, J. (2015). Humidification on Ventilated Patients: Heated Humidifications or Heat and Moisture Exchangers? The Open Respiratory Medicine Journal, 9(1), 104-111. doi: 10.2174/1874306401509010104
Hardavella, G., Karampinis, I., Frille, A., Sreter, K., &Rousalova, I. (2019). Oxygen devices and delivery systems. Breathe, 15(3), e108-e116.doi:10.1183/20734735.0204-20194
High-Flow Nasal Cannula Oxygen COVID-19 cases. (2020).https://fcmedical.co.za/wp-content/uploads/2021/01/High-Flow-Nasal-Cannula-Oxygen-Advisory-.pdf
ISO. (2021). ISO 80601-2-90:2021, Medical electrical equipment, Part 2-90: Particular requirements for basic safety and essential performance of respiratory highflow therapy equipment. International Organization for Standardization. Retrieved 8 March from https://www.iso.org/standard/80858.html
Kedzierewicz, R., Derkenne, C., Fraudin, A., Vanhaecke, P., Jouffroy, R., Jost, D., & Prunet, B. (2021). Logistical Challenge With Prehospital Use of High-Flow Nasal Oxygen Therapy in COVID-19-Induced Respiratory Distress: A Case Report. The Journal of Emergency Medicine, 61(1), 37-40.doi: 10.1016/j.jemermed.2021.03.006
Li, J., Fink, J. B., & Ehrmann, S. (2020). High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. European Respiratory Journal, 55(5), 2000892.doi:10.1183/13993003.00892-2020
Liu, X., Wu, R., Lai, L., & Lin, J. (2021). Clinical application of High-flow nasal cannula oxygen therapy in acute heart failure. Food Science and Technology.doi:10.1590/fst.40020
Nishimura, M. (2019). High-Flow Nasal Cannula Oxygen Therapy Devices. Respiratory Care, 64(6), 735-742.doi: 10.4187/respcare.06718
ResMed. (2021). Home high-flow therapy. Retrieved 8 December fromhttps://www.resmed.co.uk/healthcare-professional/respiratory-care/respiratory therapy/homehigh-flow-therapy/
SARAO. (2020). UCL-Ventura CPAP System Clinical Guidancehttps://www.sarao.ac.za/wp-content/uploads/2020/06/NVP-cpap_devices-informationsheet.pdf
Slain, K. N., Shein, S. L., & Rotta, A. T. (2017). The use of high-flow nasal cannula in the pediatric emergency department. Jornal de Pediatria, 93, 36-45.doi:10.1016/j.jped.2017.06.006
Vega, M. L., & Pisani, L. (2021). Nasal high flow oxygen in acute respiratory failure. Pulmonology., 27(3), 240-247.doi: 10.1016/j.pulmoe.2021.01.005
WHO. (2021). Technical specifications for invasive and non-invasive ventilators for COVID-19: Interim guidance, 15 April 2020. World Health Organization. Retrieved 9 December from https://apps.who.int/iris/handle/10665/331792